Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portugaliae Electrochimica Acta
versão impressa ISSN 0872-1904
Port. Electrochim. Acta vol.30 no.5 Coimbra set. 2012
https://doi.org/10.4152/pea.201205351
Kinetic Study of the Electrochemical Oxidation of Methylene Blue with Pt Electrode
M.A. El Hajj Hassan and M.M. El Jamal*
Lebanese University, Faculty of Sciences (I), Chemistry Department, El Hadath, Lebanon
Abstract
Kinetic study of the indirect oxidation of methylene blue on Pt electrode in presence of several strong electrolytes is undertaken. Different operating conditions that affected the treatment process were studied in order to find the best conditions. The order with respect to methylene blue is zero order in presence of chloride, but it is second order in presence of bromide. The oxidation rate was affected by current density, halide concentration (KCl, KBr), nature of supporting electrolyte and initial pH. However, the initial dye concentration and temperature did not show a significant effect. The oxidation of methylene blue in presence of iodide, fluoride and sulfate is absent, but it is important in presence of chloride and bromide. The product of the indirect oxidation is the chloronated (bromonated) methylene violet bernthsen.
Keywords: Pt electrode, indirect oxidation, methylene blue, effect of supporting electrolyte.
Introduction
Methylene Blue (MB+) has many uses in a range of different fields such as biology and chemistry. Photosensitizer for singlet oxygen generation, antioxidant and antiseptic, stain for fixed and living tissues, antidote to cyanide and nitrate poisoning [1]. MB+ is used as optical oxygen sensors in the food industry [1, 2]. It is an organic dye usually used to dye cotton, wool, acrylic, and silk. When it is used recklessly, it can cause serious illness such as vomiting, hard breathing, mental disorder and sweating. Different methods are being developed to carry out the elimination of toxic compounds from wastewater [3, 4]. These include biological and physicochemical methods such as flocculation combined with flotation, adsorption, membrane filtration, coagulation, and ion exchange. The literature is rich in articles about the kinetic studies of the reduction and oxidation of MB+ chemically [5-8].
In recent years, electrochemical technologies have caused great interest because they offer effective means to solve environmental problems related to industrial processes and are more adaptable to a wide range of dye wastewaters [9-12]. Electrochemical treatment of textile wastewater with a high chloride concentration employing Ti/RuO2, Ti/Pt and Ti/Pt/Ir electrodes was investigated by Polacro et al. [13]. Electrochemical methods for wastewater treatment mainly involve the direct and indirect electrochemical oxidation [14]. By indirect electrolysis, organic pollutants can be degraded by generating in situ strong oxidative intermediates that convert these pollutants into less hazardous products [15, 16]. The main oxidizing agents are active chlorine compounds, such as gaseous chlorine, hypochlorous acid, which are produced from original chlorides present in the wastewater [17]. The chemical and physical properties of chlorine in function of temperature, such as solubility and viscosity, are well studied [18]. Chlorine can electrochemically oxidize organic molecules quickly and irreversibly due to its intense oxidative activity. However, gaseous chlorine and hypochlorous acid can be easily interacted with organic molecules to form carcinogenic halogen compounds [19,20].
The efficiency of electrochemical oxidation is a function of electrode materials and supporting medium [14,17,19]. A brief review about the use of Boron Doped Diamond (BDD) in electrochemical oxidation provides total mineralization with high current efficiency of different organic molecules in real wastewaters [21]. The experimental results have indicated that the efficiency of electrochemical oxidation in chloride-mediated wastewaters is higher than that in chloride-free wastewaters for most of the electrodes [14]. The indirect oxidation of (MB+) using chloride resistant mixed oxide metal occurs by replacement of one of dimetylamino groups by oxygen accompanied by chlorination of the aromatic rings [19]. However, using other electrodes such as BDD, PbO2/TiO2 and TiRuO2/Ti oxide leads to total mineralization and decolorization of MB+ solution [14,17,22].
Therefore, in this work, a full kinetic study of the indirect oxidation of methylene blue using platinum anode in the presence of different supporting electrolytes was undertaken to know the product of the reaction in such conditions and to find the best conditions of the MB+ oxidation.
Experimental
All chemical reagents used were of analytical grade. Methylene blue (MB+Cl -) is used as purchased from BDH (82 %, C16H18N3SCl, MW: 319.85 g). Stock solution of MB+ was prepared by dissolving 100 mg in one liter of distilled water. The most electrolysis experiments are done at room temperature (293 K), in presence of 10 mg L-1 MB+, 0.1 M KX (supporting electrolyte), where X is Cl-, Br-, with 10 mA, at pH 2. The experiments were carried out in a single combined electrolytic cell. The electrolysis is performed on 100 mL solution prepared by mixing 10 mL of 100 mg L-1 of MB+, 10 mL of 0.1 M H2SO4, 10 mL of 1 M halide salt and 70 mL H2O.
The cover of the cell is designed to hold multiple electrodes. The anode and the cathode are Pt wire from Taccusel. Each electrode measured 1.2 cm length and 0.15 cm diameter. The distance between the two electrodes was 5 cm. The electrolysis is done with a Chrono-Amperostat, type CEAMD-6, from Taccusel. The rate of the indirect oxidation of MB+ was followed by measuring the absorbance at 662 nm (max. wavelength of MB+). Absorption spectra were recorded on a double beam UV-Vis Specord 200 spectrometer, Analytic Jena AG (Germany). Measurements of pH were carried out using a Schott Gerate CG 819 pH-meter.
In order to determine the effect of the experimental parameters on the observed rate constant (Kobs) and the order with respect to MB+, the absorbance (A) of the solution was recorded after a predetermined time of electrolysis, during 10 minutes at 662 nm. The plot of A, Ln A and 1/A vs. time allows to determine the best order.
The indirect oxidation of MB+ was studied in function of several parameters such as initial pH (addition of diluted solutions of H2SO4 or NaOH), nature of electrolyte, concentration of MB+, temperature, current, and ionic force. Electrochemical oxidation efficiency of MB+ was determined in the presence of several strong electrolytes such as Na2SO4, NaF, KCl, KBr, KI. No variation of pH occurred during the experiments.
Results and discussions
Visible spectroscopy analysis
The indirect oxidation of MB+ at several initial pH (2 and 8) in presence of Na2SO4 is absent, but it occurred with high efficiency in presence of chloride (or bromide) (Fig. 1a, 1b).
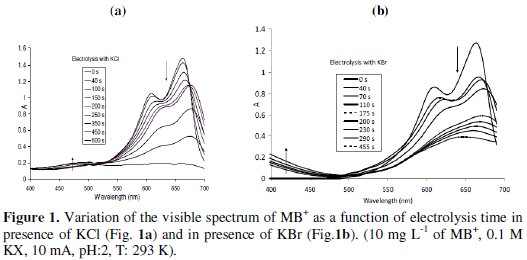
The variation of the visible spectrum with electrolysis time showed i) a decrease in the absorbance at 662 nm and a slight increase in the absorbance in the region between 450 and 500 nm, ii) a shift in λmax from 662 nm to 676 nm in the first step, then the absorbance continued decreasing with time without any shift in λmax. The presence of an isobestic point at 676 nm and the shift in λmax, prove the chlorination of the aromatic ring [23, 24]. Finally, the MB+ solution became darker with the appearance of tiny black particles in solution. The addition of CH2Cl2 or toluene to the aqueous solution let it clear and turns the organic phase pink (λmax: 544 nm) (Fig. 2).
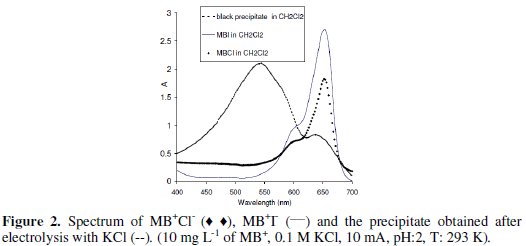
Probably the tiny black particles correspond to the chlorinated methylene violet bernthsen (MVBCl2) soluble in organic solvent but insoluble in water (uncharged compound) (Fig. 3) [25].
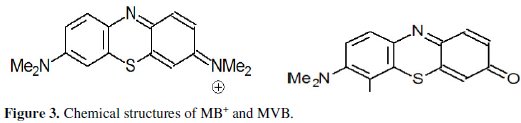
The indirect oxidation of MB+ leads to the replacement of one dimethylamino groups of MB+ with oxygen (Fig. 3). MVB is also the main product of the nucleophile attack of MB+ by OH- [26, 27]. The addition of acetone at the end the of electrolysis dissolves the dark precipitate and returns the slight black solution blue (λmax: 610 nm). Probably redox reaction occurred between the electrolysis product and acetone.
The proposed mechanism is in line with that proposed by Donaldson et al. who confirmed by several techniques (XR and LC-MS) the formation of C10H10Cl2N2OS (MVBCl2) during the anodic oxidation of MB+ [19]. In the present case, the indirect oxidation of MB+ did not lead to total mineralization of MB+ as in the case with modified TiO2 electrode [22], and TiRuO2 [17], but it produces other toxic compounds.
Kinetic Study
The order with respect to MB+ is done in presence of a large excess of (KCl), source of chlorine compounds. The rate of the reaction can be expressed as:
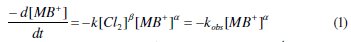
The plots of A (zero order), Ln A (1st order) and 1/A (2nd order) vs. time allow to determine the order according to the best R2 value. The experimental data are well fitted to zero-order (Fig. 4).
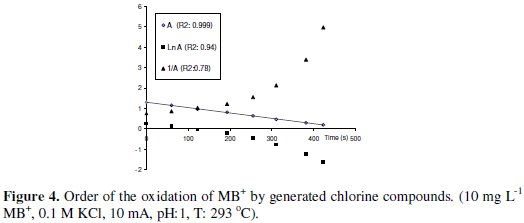
The oxidation at different concentrations of MB+ follows the same behavior and the slope of the line A vs. time remains approximately constant, so the order with respect to MB+ is zero, which means that the velocity of the reaction is independent of MB+ concentration.
Effect of strong electrolyte
The effect of the type of conductive electrolyte used in the electro degradation of toxic compounds affects strongly the efficiency of the process [22, 28]. The chloride salts give the best results since chloride plays in fact two roles: as strong electrolyte and as oxidant [29, 30]. It is interesting to study the effect of the nature of the strong electrolyte added on the oxidation rate. The electrolysis is done in presence of 0.1 M of the following supporting electrolytes: Na2SO4, NaF, KCl, KBr and KI (added separately). The results show that the decolorization in presence of Na2SO4 and NaF is absent. These results are expected since Na2SO4 and NaF are non-electro active in the conditions of the experiment, and confirm the absence of the direct oxidation of MB+ on Pt electrode.
The addition of KI to MB+ solution provokes directly its precipitation. The discoloration of MB+ solution is proportional to the volume of 1 M KI added, accompanied by the formation of a violet precipitate (MB). The dissolution of the obtained precipitate in CH2Cl2 gives light blue solution and has the same visible spectrum as MB+Cl- in CH2Cl2 with λmax at 652 nm (Figure 3). The FTIR of the violet precipitate is similar to that of MB+Cl-. In this case the electro oxidation of MB+ can not be done.
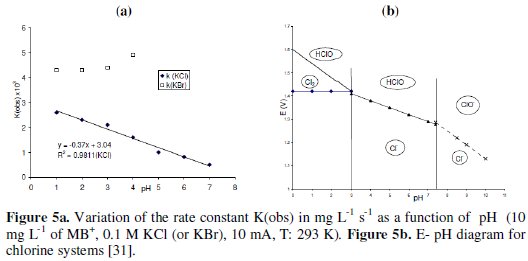
The indirect oxidation of MB+ in presence of KBr is efficient and fast as with KCl (Fig. 1b). The main differences between KCl and KBr are: i) the order with respect to MB+ in presence of KBr is 2 instead zero (in presence of KCl), ii) the rate constant k(obs) in presence of KBr remained constant for pH < 4 (Fig. 5a).
As industrial wastewater contains already high amount of chloride, and the oxidation product in presence of KBr is similar to that in presence of KCl, we focused our study in the last part on the oxidation of MB+ in presence of KCl.
Effect of pH
pH is one of the important factors that affect the performance of the electrochemical process. The effect of initial pH (pHo) on the decolorization rate of MB+ showed that the rate constant k(obs) increased linearly with decreasing in pHo for pH < 7 (Fig. 5a). Similar results are obtained during the degradation of azo dye [16] and reactive textile dyes [32]. The reason is due to the increase in HClO/Cl2 amount in acidic medium, having the HClO/Cl2 system higher standard potential than that of (ClO-/Cl-) (Fig. 5b). In basic medium (7 < pH < 9) the discoloration of MB+ is slower than that in acidic medium. For higher pH (~12) the discoloration (much slower) is due to the nucleophilic attack of OH-.
Effect of KCl concentration
Several references confirmed that the concentration of the electrolyte had a pronounced effect on the cell performance [30, 32]. The effect of KCl concentration on the decolorization rate is undertaken. Linear relation is observed between KCl concentration and the rate constant (Fig. 6a).
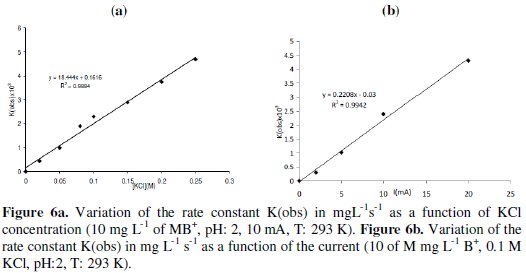
This result confirms the decolorization of the organic compounds via the generated chlorine compounds [29, 30].
Effect of current intensity
Current density is an important variable in electrochemical engineering. In this part, the indirect electro oxidation was done in acidic medium (pHo: 2) and in presence of 0.1 M KCl, at several constant currents ranging from 1 mA to 20 mA. The results show that the decolorization rate or the oxidation rate of MB+ increase linearly with the increase in current (Fig. 6b). The linearity is also observed with other organic compounds [20, 28, 30]. The increase in rate constant with the applied current is mainly the increased production of active chlorine products at the anode [17, 29].
Effect of ionic force
The effect of the ionic force on the oxidation of MB+ was investigated by varying the concentration of Na2SO4 in the medium. The result showed that the rate constant of the reaction decreases with the increase in the ionic force (Fig. 7).
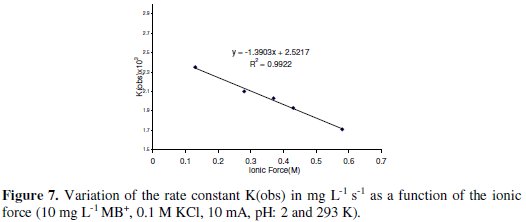
This behavior can be explained by the decrease in chlorine solubility with the increase of salt concentration [18]. Similar result is also observed with the discoloration of MB+ in presence of sulfite [25].
Effect of temperature
Table 1 presents the percent of color removal and the rate constant at different operating temperatures.

It is clear that the variation of the rate of removal of color is negligible between 283 and 300 K; however, the reaction temperature above 303 K showed a decrease in the rate of color removal. The same result is observed with other dyes [20, 22]. The decrease in Cl2 (g) solubility for T > 303 K is the major reason for the decolorization decrease [18]. Another reason may be the mass transport controlled reduction of hypochlorite according to the following cathodic loss reaction [32]:
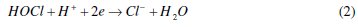
Conclusions
The discoloration of MB+ by electrochemical oxidation was affected by the operating conditions such as pH, nature of strong electrolyte and many other parameters. The order with respect to MB+ follows zero order in presence of chloride, but it is second order in presence of bromide. The efficiency of the electrochemical oxidation of MB+ was proportional to chloride concentration and current density, but inversely proportional to the initial pH and ionic force. No significant effect of temperature and MB+ concentration on the rate constant has been detected under the experimental conditions. No direct oxidation of MB+ on Pt electrode in presence of Na2SO4 and NaF. The oxidation of MB+ by electro generated chlorine and bromine produced uncharged compound which probably corresponds to chlorinate or brominate methylene blue.
References
1. Parry RT. In: Parry RT editor. Principles and Applications of Modified Atmosphere Packaging of Foods. London. Blackie Academic Press: 1993. [ Links ]
2. http://en.wikipedia.org/wiki/Methylene blue [ Links ]
3. Robinson T, McMullan G, Marchant R, Nigam P. Bioresour Technol. 2001;77:247. [ Links ]
4. Ong S-T, Keng P-S, Lee W-N, Ha S-T, Hung Y-T. Water. 2011;3:157.
5. Katafias A, Kita P, Wrzeszcz G. Trans Metal Chem. 2006;32:31. [ Links ]
6. Pande S, Ghosh SK, Nath S, Praharaj S, Jana S, Panigrahi S, Basu S, Pal T. J Colloid Interface Sci. 2006;299:421.
7. Pietkiewicz-Graczyk A, Impert O, Katafias A, Kita P. Polish J Chem. 2003;77:475. [ Links ]
8. Impert O, Katafias A, Kita P, Mills A, Pietkiewicz-Graczyk A, Wrzeszcz G. Dalton Trans. 2003:348.
9. Santos V, Morao A, Pacheco MJ, Ciriaco L, Lopes A. J Environ Eng Manage. 2008;18:193.
10. Martinez-Huitle CA, Ferro S. Chem Soc Rev. 2006;35:1324. [ Links ]
11. Peralta-Hernandez JM, Mejia S, Godinez LA, Meas-Vong Y. Environ Research. M. Palomar, editor. India: Research Signpost, Kerala; 2005. [ Links ]
12. Palma-Goyes RE, Guzmn-Duque FL, Peuela G, Gonzlez I, Nava JL, Torres-Palma RA. Chemosphere. 2010;81:26.
13. Polacro M, Palmas S, Renoldi F, Mascia M. J Appl Electrochem. 1999;29:147. [ Links ]
14. Wu M, Zhao G, Li M, Liu L, Li D. J Haz Mat. 2009;163:26.
15. Abu Ghalwaa NM, Abdel-Latif MS. J Iranian Chem Soc. 2005;2:238. [ Links ]
16. Alameddine I, El Jamal MM. J Univ Technol Metall (Sofia). 2009;44:127. [ Links ]
17. Panizza M, Barbucci A, Ricotti R, Cerisola G. Sep Purif Technol. 2007;54:382. [ Links ]
18. Schmittinger P. Chlorine. In: Ullmann's Encyclopaedia, vol. 6A. Weinheim: VCH; 1986. P. 399.
19. Donaldson JD, Grimes SM, Yasri NG, Wheals B, Parrickand J, Errington WE. J Chem Technol Biotechnol. 2002;77:756.
20. Raidan RI, Tabbara A, El Zant AA, El Jamal MM. J Univ Tech Metall (Sofia). 2009;44:79. [ Links ]
21. Peralta-Hernandez JM, Mendez-Tovar M, Guerra-Sanchez R, Martinez-Huitle CA, Nava JL. Int J Electrochem. 2012:Article ID 154316.
22. Abu Ghalwa NM, Zaggout FR. J Environ Sci Health A. 2006;41:2271. [ Links ]
23. Korbahti BK, Tanyolac A. Water Res. 2003;37:1505. [ Links ]
24. Plater MJ. Arkivoc. 2003:37. [ Links ]
25. Mills A, Wang J. J Photochem Photobiol A. 1999;127:123. [ Links ]
26. Katafias A, Kita P, Wrzeszcz G. Trans Metal Chem. 2006;32:31. [ Links ]
27. Zaggout FR, Abu Ghalwa N. J Environ Manag. 2008;86:291. [ Links ]
28. Parsa JB, Abbasi M. Acta Chim Slov. 2007;54:792. [ Links ]
29. Zaviska F, Drogui P, Blais J-F, Mercier G. J Appl Electrochem. 39 (2009) 2397.
30. Maurice M, Desbarres J, Colin C, Jardy A, Bauer D. Chimie des solutions. Techniques et Documentation; 1990.
31. Kariyajjanavar P, Jogttappa N, Nayaka YA. J Haz Mat. 2011;190:952. [ Links ]
32. Rajkumar D, Kim JG. J Haz Mat. 2006;B136:203. [ Links ]
Acknowledgements
The authors wish to thank the ''Ecole Doctorale de Sciences et Technologie'' in Lebanese University for its help.
*Corresponding author. E-mail address: mjamal@ul.edu.lb
Received 21 October 2012; accepted 31 October 2012














