Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portugaliae Electrochimica Acta
versão impressa ISSN 0872-1904
Port. Electrochim. Acta vol.29 no.4 Coimbra 2011
Corrosion Behaviour of Stainless Steel 304 Electroplated with Zinc Followed by Blue Passivation
H.B. Sherine*,1, C.C. Rajakumari1 and S. Rajendran2
1Department of Chemistry, Holy Cross College, Tiruchirappalli - 620 002, Tamilnadu, India
2Department of Chemistry, GTN Arts College, Dindigul - 624 005, Tamilnadu, India
Abstract
The corrosion resistance of three stainless steel materials, namely, stainless steel (SS), stainless steel electroplated with zinc (SS-Zn) and stainless steel electroplated with zinc followed by blue passivation (BP), has been evaluated in an aqueous solution containing 3.5% NaCl. A potentiodynamic polarization study and AC impedance spectra have been used to investigate the corrosion behaviour of these metals. The corrosion resistance of these materials in 3.5% NaCl increased in the following order: SS>SS+Zn+BP>SS+Zn.
Keywords: decolourisation, corrosion prevention, electroplating, blue passivation.
Introduction
The development of the chemical, fertilizer, petrochemical, refining and energy industries depends in most cases, on resolving the problems associated with the use and maintenance of stainless steel [1]. The most important problem faced with the use of stainless steel is intergranular corrosion (IGC). The IGC resistant stainless steel was achieved by stabilizing the characteristics of the steel decreasing its percentage of carbon to a very low level [2]. Characterization of oxide films formed on metals and alloys has been subject of study for many years, because the physical and chemical properties of oxide films can alter the mechanism and kinetics of the corrosion processes [3-5]. Particularly, the characteristics of oxide films formed on type 304 stainless steel and carbon steel as nuclear power plant materials have been subject of investigations, to understand environment related materials failure problems. Intergranular stress corrosion cracking (IGSCC) of Type 304 stainless steel has been a major concern in boiling water reactors undergoing normal water containing 100 to 300 ppb of oxygen, 200 to 500 ppb of H2O2 and < 10 ppb of hydrogen. Self-organized porous structures produced by anodization of metals or semiconductors have attracted much attention regarding applications [6-14]. The composite electroplating allows to co-deposit fine particles of metallic or non-metallic compounds into plated layers to improve the surface properties and compare the performance of pure nickel and Ni-SiC nanostructured composite coatings, and the results indicated that the co-deposition of nickel and SiC nano particles leads to uniform deposits possessing better abrasion, wear and corrosion properties [15].
The present study is undertaken (i) to electroplate zinc on stainless steel surface in an electroplating unit, using zinc anode and a bath containing zinc chloride, potassium chloride and boric acid; (ii) to do blue passivation on the zinc plated carbon steel surface; (iii) to study the corrosion resistance behaviour of the above electroplated surface (a) by immersing it in an aqueous solution containing 3.5 % of NaCl, (b) by placing a drop of CuSO4 solution on the metal surface, and (c) by polarization study, AC impedance spectroscopy and cyclic voltammetry; and (iv) to decolourise methyl orange solution using (a) stainless steel, (b) stainless steel electroplated with zinc, and (c) stainless steel electroplated with zinc followed by blue passivation.
Experimental
Preparation of the specimen
Three metal specimens, namely, stainless steel (SS 304), stainless steel coated with zinc, and stainless steel coated with zinc followed by blue passivation, were chosen for the present study. Stainless steel and stainless steel coated with zinc are compared with stainless steel coated with zinc followed by blue passivation. The composition of SS 304 was (wt%) 10 Cr, 8 Ni, 0.80 Mn, 0.044 C, 0.0025 P, 0.0015 S and the balance iron [16], and specimens with the dimensions of 1.0×4.0×0.2 cm were used for electroplating and to measure the corrosion resistivity of the metal surface by weight loss method.
Method of electrodeposition
The process of electroplating the stainless steel specimens involves pickling with con. HCl (16 N), washing with distilled water, drying, polishing, degreasing with cleaning powder containing soda, chalk and nice emery powder, drying and immersing in bath solution. The composition of the bath solution for zinc plating was ZnCl2 (100 g/L), KCl (225 g/L), H3BO3 (40 g/L). For electrodeposition, pure zinc plate acts as anode and stainless steel specimen acts as cathode. The process was carried out at room temperature (35 ºC) and no agitation of the bath solution is required. DC current was passed for the required time (5 minutes). After electrodeposition the specimen was washed with water and dried [17]. The composition of the post treatment bath solution for blue passivation was 200 g of sodium dichromate and 5 g of KCr(SO4)2.12H2O (chrome alum). 4 g of this mixture were dissolved in 10 mL of nitric acid and made upto 1 litre with water. The zinc plated stainless steel was immersed in the blue passivation bath for one minute. Then the metal specimens were dried.
Measurement of corrosion resistance of the electroplated metal surface
The following studies were used to measure the corrosion protective nature of the film formed on the stainless steel surface after electroplating.
Immersion in chloride environment
The metal specimens in triplicate were immersed in 100 mL of an aqueous solution containing 3.5% of NaCl for a period of one day. The weights of the specimens before and after immersion were determined using a digital balance.
The corrosion rate (CR) was calculated using the equation
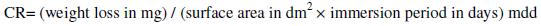
Action of 1% copper sulphate solution
One drop of 1% copper sulphate solution was placed on the surface of the metal. The time taken for the formation of a red solution was measured, because it is an indication of the rate of electron transfer (corrosion process) from the iron to the Cu2+ ion on the metal surface.
Potentiodynamic polarization study
Polarization study was carried out in H&CH electrochemical workstation impedance Analyzer Model CHI 660A provided with iR compensation facility, using a three electrode cell assembly. Stainless steel(SS), stainless steel coated with zinc (SS+Zn), or stainless steel electroplated with zinc followed by blue passivation (SS+Zn+BP), were used as working electrode, platinum as counter electrode and saturated calomel electrode (SCE) as reference electrode. The corrosion parameters such as linear polarization resistance (LPR), corrosion potential (Ecorr)), corrosion current( Icorr) and Tafel slopes (bc and ba) were calculated.
Alternating current impedance spectra
AC impedance spectra were recorded in the same instrument used for polarization study, using the same type of three electrode cells assembly. The real part (Z') and the imaginary part (Z'') of the cell impedance were measured in ohms for various frequencies. The impedance values [log (z/ohm)] were derived from Bode plots.
Cyclic voltammetry
Cyclic voltammograms were recorded with the cell set up used for polarization study. The scan rate was 0.1 V/s. The graph between potential (V) vs. current (A) was plotted.
Decolourisation process
Decolourisation of a dye such as methyl orange was attempted using various electrodes such as stainless steel, stainless steel electroplated with zinc and stainless steel electroplated with zinc followed by blue passivation. The optical density of the methyl orange solution before and after decolourisation was measured by an instrument photoelectric colorimeter - 112. The electrodes were immersed in 100 mL of the solution containing 50 ppm of methyl orange. The solution was subjected to electrochemical decolourisation process after addition of various concentrations of NaCl. Graphite was used as cathode. Stainless steel, electroplated stainless steel, or stainless steel electroplated with zinc followed by blue passivation, were used as anode. The electrolysis was carried out in an undivided cell with a stirring bar.
The % of decolourisation efficiency (DE) was calculated using the relation
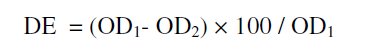
where OD1 and OD2 are optical densities before and after decolourisation, respectively.
Results and discussion
Analysis of results of weight loss method
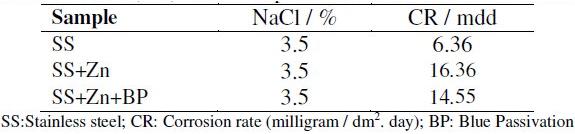
Corrosion rates obtained by weight loss method of stainless steel (SS) samples, before and after electroplating, in an aqueous solution containing 3.5% NaCl, and of stainless steel electroplated with zinc followed by blue passivation, are given in Table 1. It is observed that when stainless steel is electroplated with zinc, the corrosion rate increased from 6.36 mdd to 16.36 mdd. This suggests that the corrosion resistance of stainless steel decreases after zinc plating. This is due to the fact that the zinc film is broken in presence of 3.5% NaCl. Hence corrosion is accelerated. Further, when stainless steel 304 and zinc are in contact, zinc becomes anode and hence it undergoes corrosion [18]. But for stainless steel electroplated with zinc followed by blue passivation, the corrosion rate decreased from 16.36 mdd to 14.55 mdd. The corrosion rates presented in Table 1 reveal that the blue passivated stainless steel is more corrosion resistant than zinc coated stainless steel, but it is less corrosion resistant than the stainless steel itself.
Table 1. Corrosion rates (CR) of metal specimens immersed in 3.5% NaCl solution.
Action of 1% copper sulphate solution on the metal surface
The time for the appearance of reddish brown solution, when a drop of 1% copper sulphate solution was placed on the metal surface, before and after electroplating, is given in Table 2.
Table 2. Time required for the appearance of reddish brown deposit when one drop of 1% CuSO4 solution was placed on the metal surface before and after electroplating.

Usually, when a drop of 1% copper sulphate solution is placed on polished carbon steel surface (CS), a red colour appears. The formation of red colour is due to the conversion of blue Cu2+ ion into Cu which is red in colour.
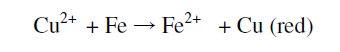
When one drop of 1% copper sulphate was placed on the carbon steel surface, a red solution appeared within 65 seconds. This is due to the fact that blue Cu2+ ion is reduced to red Cu because of electron transfer from Fe to Cu2+.
When one drop of 1% copper sulphate solution was placed on stainless steel surface, red colour did not appear even after 1200 seconds. But blue colour disappeared immediately. A colourless solution was obtained.
This may be explained by the fact that there is formation of colourless Cu+ ion. This Cu+ ion is produced by interaction of blue Cu2+ ion and the one electron that comes from the metal surface. This electron may come from Ni or Cr or Fe present in the stainless steel 304.
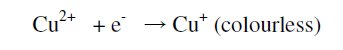
Similarly, when one drop of 1% copper sulphate was placed on stainless steel surface coated with zinc, red colour did not appear even after 1200 seconds. But blue colour disappeared immediately. A colourless solution was obtained.
This may be explained by the fact that there is formation of colourless Cu+ ion. This Cu+ ion is produced by interaction of blue Cu2+ ion and the one electron that comes from the metal surface. This electron may come from Ni or Cr or Fe or Zn present in the stainless steel 304 or zinc coated stainless steel.
Similarly, when one drop of 1% copper sulphate was placed on stainless steel surface coated with zinc followed by blue passivation, red colour did not appear even after 1200 seconds. But blue colour disappeared at 185th second. A colourless solution was obtained. This is because when zinc is electroplated on stainless steel, zinc becomes less noble and it undergoes corrosion and stainless steel is protected [18].
Analysis of potentiodynamic polarization study
The polarization curves of stainless steel (SS), stainless steel electroplated with zinc, and stainless steel electroplated with zinc followed by blue passivation immersed in an aqueous solution containing 3.5% NaCl, are shown in Fig. 1a, 1b and 1c. The corrosion parameters such as corrosion potential (Ecorr), Tafel slopes (bc=cathodic) (ba=anodic), linear polarization resistance (LPR) and corrosion current (Icorr), are given in Table 3. When stainless steel was immersed in an aqueous solution containing 3.5% NaCl, the corrosion potential was -198 mV vs. SCE. When stainless steel was electroplated with zinc, the corrosion potential was shifted to cathodic side -1043 mV. This is due to the deposition of Zn on SS surface [17] so that metal surface becomes more active and hence undergoes corrosion. The LPR value decreases from 503.9×101 ohm cm2 5.128×10 ohm cm2 for SS 304 coated with zinc. The corrosion current increases from 6.827×10-6 to 636.0×10-6 A/cm. Decrease in LPR value and increase in the corrosion current suggest that the effective protective film had not been formed on stainless steel electroplated with zinc [19-20]. These observations suggest that the corrosion protecting efficiency decreases when SS is electroplated with Zn and the corrosion rate increases. This suggests that zinc film coated on SS is easily broken when in 3.5% NaCl solution. This enhances the corrosion rate of iron in SS, as sodium chloride solution enters into the pores created by the breaking of zinc film coated on SS surface.
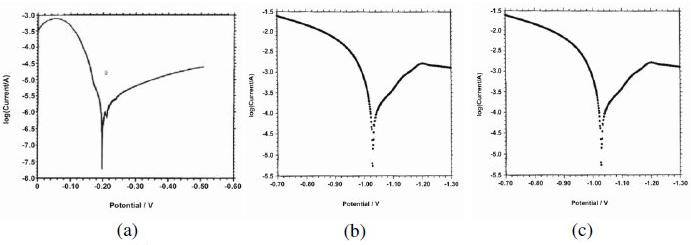
Figure 1. Polarisation curves of stainless steel (a) without further treatment, (b) electroplated with zinc and (c) electroplated with zinc followed by blue passivation, immersed in an aqueous solution containing 3.5% NaCl.
Table 3. Corrosion parameters of stainless steel (SS) samples immersed in an aqueous solution containing 3.5% NaCl, before and after electroplating. (Obtained from potentiodynamic polarization study).
When stainless steel electroplated with zinc followed by blue passivation was immersed in an aqueous solution containing 3.5% NaCl, the corrosion potential was -1028 mV, which is cathodic when compared to stainless steel, but it is slightly anodic when compared with zinc coated stainless steel. The LPR value of stainless steel electroplated with zinc followed by blue passivation was 9.238×101 ohm cm2. The corrosion current value for stainless steel electroplated with zinc followed by blue passivation increased to 369.7×10-6 A|cm2. The decrease in the LPR value and the increase in the corrosion current suggest that the blue passivated stainless steel is more corrosion resistant than the stainless steel electroplated with zinc; but it is less corrosion resistant than stainless steel itself. The corrosion resistance of SS is better than SS coated with zinc, and than SS coated with zinc followed by blue passivation, as revealed by higher LPR value and lower current.
Hence, it is suggested that stainless steel coated with zinc should not be used in coastal area, ships and shipyards, and also for storing thiourea in industries [17]. Otherwise machines, made of this combination will undergo corrosion because of the chloride vapours present in coastal area.
Analysis of AC impedance spectra
The AC impedance spectra (Bode plots) of stainless steel electroplated with zinc followed by blue passivation immersed in an aqueous solution containing 3.5% NaCl are shown in Fig. 2a, 2b and 2c. The real impedance [log (z/ohm)] values are given Table 4. It was observed that when stainless steel was immersed in 3.5% NaCl solution the real impedance value was 2.05. After zinc deposition this value decreased to 1.46. For blue passivated stainless steel the real impedance value was 1.49.
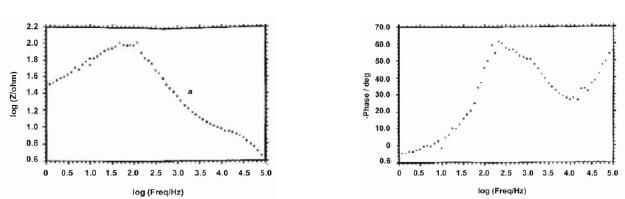
Figure 2a. AC impedance spectra of stainless steel immersed in an aqueous solution containing 3.5% NaCl (Bode plot).
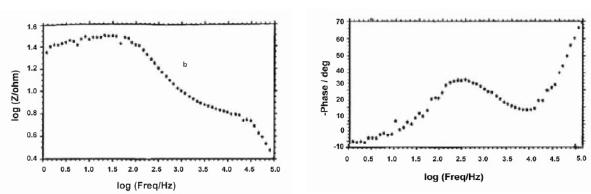
Figure 2b. AC impedance spectra of stainless steel electroplated with zinc immersed in an aqueous solution containing 3.5% NaCl (Bode plot).
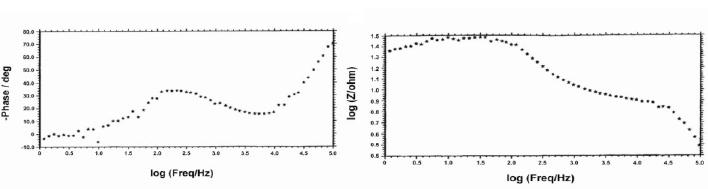
Figure 2c. AC impedance spectra of stainless steel electroplated with zinc followed by blue passivation immersed in an aqueous solution containing 3.5% NaCl (Bode plot).
Table 4. AC impedance parameters of stainless steel (SS) samples immersed in an aqueous solution containing 3.5% NaCl, before and after electroplating (obtained from Bode plot).

The decrease in the real impedance value for electroplated (Zn) stainless steel, 1.46, when compared to stainless steel, 2.05, shows that corrosion is accelerated in the case of zinc plated stainless steel; but the value 1.49 for blue pasivated stainless steel suggests that the blue passivated stainless steel is more corrosion resistant than zinc coated stainless steel, but less corrosion resistant than the stainless steel itself.
Analysis of cyclic voltammograms
The cyclic voltammograms of stainless steel, stainless steel electroplated with zinc, and stainless steel electroplated with zinc followed by blue passivation, immersed in an aqueous solution containing 3.5% NaCl, are shown in Fig. 3a, 3b and 3c. It was observed that redox couples were absent in these cyclic voltammograms.
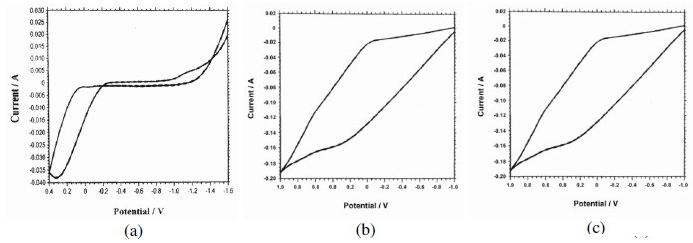
Figure 3. Cyclic voltammograms of stainless steel (a) without further treatment, (b) electroplated with zinc and (c) electroplated with zinc followed by blue passivation, immersed in an aqueous solution containing 3.5% NaCl.
Decolourisation process
Decolourisation using stainless steel
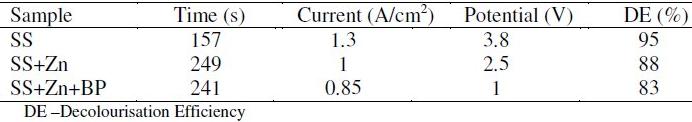
The efficiency of decolourisation of methyl orange (50 ppm) is given in Table 5. Uncoated stainless steel anode and graphite cathode were immersed in the solution to decolourise 50 ppm of methyl orange solution. The solution was electrolysed for 10 minutes without addition of NaCl. There was no decolourisation. The experiment was repeated after addition of 7 g of NaCl and the solution was electrolysed. The current density was 1.3 A /cm2 and the potential was 3.8 Volts. Methyl orange solution was completely decolourised within 157 seconds. The efficiency of decolourisation was 95%.
Table 5. Efficiency of decolourization of methyl orange (50 ppm). Optical density for methyl orange = 0.42.
Decolourisation using stainless steel electroplated with zinc
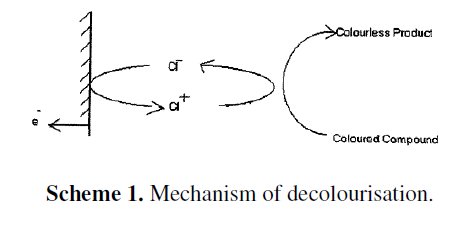
The experiment was repeated by using stainless steel electroplated with zinc after addition of 7 g of NaCl; the solution was electrolysed. The current density was 1 A/cm2 and the potential was 2.5 Volts. Methyl orange solution was decolourised within 249 seconds. The efficiency of decolourisation was 88%. When NaCl solution was electrolysed, the active species produced is Cl+ [21-23]. This oxidized the coloured material into colourless product. (Scheme1).
Decolourisation using stainless steel electroplated with zinc followed by blue passivation
The experiment was repeated by using stainless steel electroplated with zinc and blue passivation, after addition of 7 g of NaCl. The solution was electrolysed. The current density was 0.85 A/cm2 and the potential was 1 Volt. Methyl orange solution was decolourised within 241 seconds. The efficiency of decolourisation was only 83%.
The decolourisation efficiency is in the order SS>SS+Zn>SS+Zn+BP. This suggests that the ease of electron release from the metal surface is in the order SS>SS+Zn>SS+Zn+BP.
Conclusions
Zinc was electro deposited on stainless steel 304 surface using the bath containing ZnCl2, KCl and boric acid. Then blue passivation was done. The corrosion protective efficiency of the film was evaluated by weight loss method, copper sulphate test, polarization and AC impedance studies. The zinc deposited stainless steel was used to decolourise methyl orange solution.
When uncoated stainless steel 304 was immersed in an aqueous solution containing 3.5% NaCl, the corrosion rate was 6.36 mdd. But when zinc was deposited in SS 304, the corrosion rate increased to 16.36 mdd, but for blue passivated SS 304 the corrosion rate decreased to 14.55 mdd. This shows that SS 304 electroplated with zinc followed by blue passivation is more corrosion resistant than zinc electroplated SS 304.
Polarization study and AC impedance spectra lead to conclusion that when stainless steel is electroplated with zinc, corrosion is accelerated and corrosion protective efficiency was in the order SS>SS+Zn+BP>SS+Zn.
The metal specimens were used to decolourise 50 ppm of methyl orange solution and the decolourising efficiency was in the order SS>SS+Zn>SS+Zn+BP.
Hence stainless steel coated with zinc should not be used in coastal area, in ships and shipyards.
References
1. M.K. Karfoul, ''Industrial Corrosion and Corrosion Control Technology'', H.M. Shalaby, A. Al-Hashem, M. Lowther, J. Al-Besharak (Eds.), Kuwait Inst. Sci. Research, Safat, 1996. p. 431-439. [ Links ]
2. E.C. Bain, R.H. Aborn, J.J.B. Rutherford, ''The Nature and Prevention of Intergranular Corrosion in Austenitic Stainless Steel'', Trans. Am. Soc. Steel Treating, 1993. Vol.XXI, No.1, p.481-509. [ Links ]
3. E.C. Potter, G.M.W. Mann, ''Oxidation of Mild Steel in High Temperature Aqueous System'', 1st Int. Congress on Metallic Corrosion'', 10-15 April (1961), p. 417. [ Links ]
4. G.M.W. Mann, ''High -temperature, High-pressure Electrochemistry in Aqueous Solutions'', Tx: NACE International, Houston, (1976), p.36. [ Links ]
5. L. Tomlinson, Corrosion 37 (1981) 591. [ Links ]
6. H. Tsuchiya, M. Hueppe, T. Djenizian, P. Schmuki, Appl. Surf. Sci. 547 (2003) 268. [ Links ]
7. H. Tsuchiya, M. Hueppe, T. Djenizian, P. Schmuki, S. Fujimoto, Sci.Tech. Adv.Mater. 5 (2004) 195.
8. H. Tsuchiya, P. Schmuki, J. Electrochem. Comm. 7 (2005) 49-52. [ Links ]
9. D. Gong, C.A. Grimes, O.K. Varghese, W. Hu, R.S. Singh, Z. Chen, E.C. Dickey, J. Mater. Res.16 (2001) 3331.
10. V. Zwilling, E. Darque-Ceretti, A. Boutry-Forveille, Electrochim. Acta 45 (1999) 921. [ Links ]
11. S.S. Chang, S. Kurokawa, A. Sakai, Appl. Surf. Sci. 217 (2003) 50. [ Links ]
12. S.S. Chang, C.H. Park, S.W. Park, Mater. Chem. Phys. 79 (2003) 9. [ Links ]
13. H.C. Shin, J. Dong, M. Liu, Adv. Mater.16 (2004) 237. [ Links ]
14. R. Beranek, H. Hildebrand, P. Schmuki, Electrochem. Solid-state Lett. 6 (2003) 312. [ Links ]
15. A.M. Lekka, A.N. Koulombi, B.M. Gajo, J.P.L. Bonora, Electrochim. Acta 50 (2005) 4551-4556. [ Links ]
16. I. Gurappa, Mater. Charact. 49 (2002) 73-79. [ Links ]
17. S. Rajendran, V. Agnes Brigita, J. Manivannan, J. Jeyasundari, Port. Electrochim. Acta 27 (2009) 555-564. [ Links ]
18. G.M. Fontana, ''Corrosion Engineering'', Mc Graw-Hill, 3rd Ed., New York, 2006. p.43. [ Links ]
19. S. Rajendran, J. Paulraj, P. Rengan, J. Jeyasundari, M. Manivannan, J. Dentistry Oral Hygiene 1 (2009) 1-8.
20. S. Rajendran, V. Uma, A. Krishnaveni, J. Jeyasundari, B. Shymaladevi, M. Manivannan, Arab. J. Sci. Eng. 34 (2009) 147-158.
21. J. Sathiyabama, S. Rajendran, J.Arockiaselvi, Bull. Electrochem. 22 (2006) 363-369. [ Links ]
22. S. Rajendran, D.C. Trivedi, J. Synth. 2 (1995) 153. [ Links ]
23. R.L. Doston, R.W. Lynch, J. Electrochem. Soc. 128 (1981) 798. [ Links ]
*Corresponding author. E-mail address beni2@rediffmail.com
Received 3 December 2010; accepted 12 March 2011
Acknowledgement
The authors are thankful to their respective managements, St. Joseph's Research and Community Development Trust, Dindigul and University Grants commission, India, for their help and encouragement.














