Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portugaliae Electrochimica Acta
versão impressa ISSN 0872-1904
Port. Electrochim. Acta v.28 n.5 Coimbra 2010
Electrochemical Reduction behaviour of Donepezil at β-Cyclodextrin Modified Carbon Paste Electrode
C.N. Rao,1 K. Subbarayudu,1 C.N. Rao,2 P. Venkateswarlu1,*
1 Dept. of Chemistry, Sri Venkateswara University, Tirupati-517 502, India
2 Dept. of Zoology, Sri Venkateswara University, Tirupati-517 502, India
DOI: 10.4152/pea.201005349
Abstract
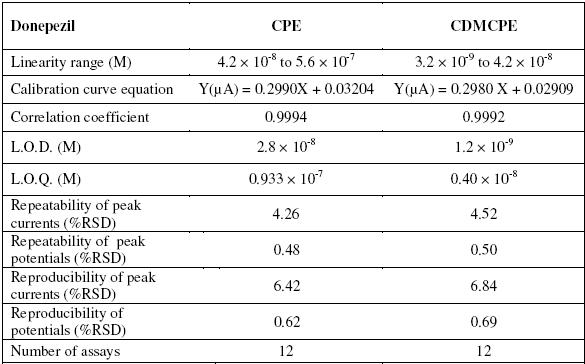
A simple, accurate and sensitive voltammetric method for determination of donepezil (DNZ) using β-cyclodextrin modified carbon paste electrode (CDMCPE) is developed. CDMCPE exhibited significantly increased sensitivity and selectivity for DNZ compared to the bare carbon paste electrode (CPE). Effect of accumulation potential, accumulation time, pH of buffer, etc., on peak currents for the determination of DNZ was studied using cyclic voltammetry (CV) and differential pulse voltammetry (DPV). Peak currents showed a linear response in the concentration range 4.2 x 10-8 to 5.6 x10-7 M and 3.2 x 10-9 to 4.2 x 10-8 M at CPE and CDMCPE respectively. Limit of detection (LOD) and limit of quantification (LOQ) were found to be 1.2 x 10-9 and 0.40 x 10-8 M. The proposed method has been successfully applied for the determination of DNZ in spiked urine samples, serum samples and pharmaceutical formulations.
Keywords: donepezil, β-cyclodextrin, cyclic voltammetry, differential pulse voltammetry, pharmaceutical formulations, urine samples.
Introduction
Donepezil (2-[(1-benzyl-4-piperidyl) methy] -5, 6-dimethoxy-2,3-dihydroinden-1-one) is a centrally acting reversible acetylcholinesterase inhibitor. Its main therapeutic use is in the treatment of Alzheimer's disease where it is used to increase cortical acetylcholine. Donepezil hydrochloride is postulated to exert its therapeutic effect by enhancing cholinergic function. This is accomplished by increasing the concentration of acetylcholine through reversible inhibition of its hydrolysis by acetylcholinesterase [1-3]. Donepezil was determined by several analytical methods such as HPLC coupled with ultra violet detector [4-10], fluorescence detector [11, 12], and single mass spectrometry detector [13].
However there is a drawback of insufficient selectivity in the aforementioned procedures for the determination of donepezil.
Modified carbon paste electrodes are widely used in analytical field. The cyclodextrins (CDs) are important and widely studied examples of host molecules which are capable of forming inclusion complexes with a variety of guests by incorporating them within the datively non-polar cavity [14-15]. β -cyclodextrins are cyclic carbohydrates with seven glucose units, which can capture the carbonyl group. The mole ratio of the guest to host (CDs) is usually 1:1 and 2:1; due to these reasons, there has been increasing interest in the use of organic electrode reactions [16]. Reddy et al. reported Electrochemical determination of sparfloxacin in pharmaceutical formulations and Urine samples using a β-cyclodextrin modified carbon paste electrode [17]. The complexation abilities and analytical applications of working electrodes modified with CDs are reviewed by Labuda Jan [18]. Bersier et al. [19] reviewed the electrochemical behavior of CDs and CD-inclusion complexes. The electrochemical reduction behavior is a better way for the determination of biological compounds compared to the other methods [24-27]. We reported voltammetric methods for the determination of several pharmaceutical compounds [28-32].
The purpose of the present work is to construct a sensitive and selective analytical method to determine donepezil in a simple, fast and inexpensive way. In this work bare carbon paste electrode (CPE) and β-cyclodextrin modified carbon paste electrodes (CDMCPE) were used as working electrodes.
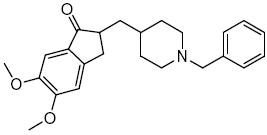
Figure 1. Structure of donepezil.
Experimental
Electrochemical apparatus, chemicals and reagents
Voltammograms were recorded with a Metrohm 757 VA computrace (Herisau, Switzerland). A model Metrohm 632-pH meter was used to carry out the pH measurements. The modifier β- cyclodextrin was purchased from Fluka. Paraffin oil and graphite powder (l-2 particle size) from Aldrich. Donepezil is purchased from Sigma. Stock solutions are prepared in methanol and kept at dark place. The supporting electrolyte (Britton-Robinson buffer) is prepared using 0.04 M orthophosphoric acid (H3PO4), acetic acid (CH3COOH) and boric acid (H3BO3); pH is adjusted by the addition of 0.2 M sodium hydroxide (NaOH) solution. Standard solutions are prepared on dilution of the stock solution with triple distilled deionized water. All the chemicals used for the preparation of solutions and supporting electrolytes are reagent grade.
Recommended procedure
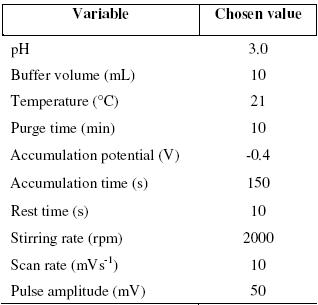
An appropriate amount of analyte and supporting electrolyte (BRB) is taken into 50 mL electrolytic cell and purged with oxygen -free nitrogen for 10 min (and for 2 min in subsequent runs). The voltammograms are recorded after small aliquot of the standard solution is added and then voltammograms recorded after each addition under similar conditions. The required accumulation potential of -0.4 V is then applied to the electrodes (hand made CPE or CDMCPE), with a stirring speed at 2000 rpm. The stirring is stopped and after 10 sec of rest, the voltammograms are recorded by scanning the potential towards the negative direction. All the measurements are performed at room temperature 21 ±3 ºC.
Effect of pH
The peak current increases gradually with the increase of pH of the solution until it reaches the maximum value at pH 3.0; but for pH values higher than 3, peak current decreases. As the pH is increased, a more negative value of peak potential is observed. It indicates the existence of a protonation reaction for the reduction of donepezil. The experimental results show that the shapes of curves are nearly the same in all cases; however, the current intensity in the BR buffer is higher than that of acetate and phosphate buffers. Hence BR buffer of pH 3.0 is selected for further experimental studies.
Effect of accumulation potential
The dependence of the peak currents on the accumulation potential was studied gradually over the range at -0.1 to -0.8 V for 3.0x10-9 M of donepezil at bare CPE and CDMCPE in BR buffer solution of pH 3.0 and an accumulation period of 300 s (bare CPE) and 150 s (modified CDMCPE). The developed peak current is achieved at maximum potential of -0.4 V.
Effect of accumulation time
The effect of stripping peak currents on the accumulation time period is determined in BR buffer of pH 3.0 for 3.0x10-9 M donepezil. The stripping peak at CDMCPE increases linearly with an increase in the accumulation time up to 150 s. As the time becomes longer than 150 s, the peak current shows deviation from linearity, indicating that complete coverage of the analyte on the surface of CDMCPE is obtained. Hence, the preconcentration time at bare CPE is 300 s and at CDMCPE 150 s. The maximum peak current is observed at CDMCPE and moreover, the uptake of the drugs at CDMCPE is faster than that of bare CPE. The accumulation time of 150 s at CDMCPE is chosen for the compound for all further studies.
Effect of other parameters
The other experimental parameters, those that directly affect the voltammetric response, such as scan rate, stirring rate, pulse amplitude, and rest period, are optimized. It is found that these parameters have pronounced effects on the peak current response, so the working conditions decided upon are a stirring rate 2000 rpm, a scan rate 10 mV/s and pulse amplitude 50 mV. When the scan rate and pulse amplitude are increased, the increase in peak width is observed. So, low scan rates of 10 mV/s and pulse amplitude of 50 mV are found to be suitable for experimental studies. The different portions of β-cyclodextrin in the carbon paste are examined to get an improved signal. The most well defined signal is achieved with a composition of 64% β-cyclodextrin. For higher composition of the β-cyclodextrin, residual currents increase and for lower ones, the peak currents decrease; all the experimental conditions are given in table 1.
Table 1. Chosen experimental conditions.
Calibration studies by DPAdSV
The electrochemical reduction of donepezil is carried out by DPV at CDMCPE. The dependence of the DPV peak current on donepezil concentration for an accumulation time of 150 s at -0.4 V in BR buffer, pH 3.0, shows a linear relationship from 3.2 x 10-9 M to 4.2 x 10-8 M (DNZ) with Y (µA) = 0.2980 X + 0.02909 at CDMCPE. The LOD (limit of detection) and LOQ (limit of quantification) are calculated using the equations, LOD = 3 S.D. / M. Here S.D. is the standard deviation of the peak currents and M is the slope of the calibration curve. The precision of the methods are evaluated by repeating five experiments on the same day in the same standard solution (repeatability) and over 5 days from the different standard solutions and different electrodes of same composition (reproducibility) repeating the experiments for 10 times. To study these experiments the chosen concentration of the solution is 3.0 x 10-9 M. The statistical parameters are shown in Table 2. Stock solutions of DNZ show the same peak current values without any change; this is even after a month, which confirms stability of the above used drug.
Table 2. Experimental data of donepezil.
Results and discussion
Cyclic voltammetry
Fig. 2 represents the cyclic voltammograms for 3.0x10-9 M donepezil in BR buffer solution of pH 3.0 at bare carbon paste electrode (CPE) and β-cyclodextrin modified carbon paste electrode (CDMCPE), with accumulation times of 300 s and 150 s, respectively, accumulation potential of -0.4 V.
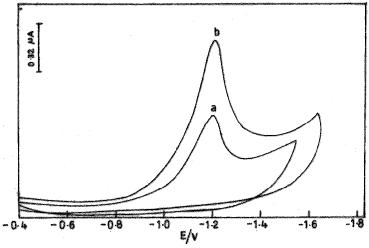
Figure 2. Typical CV of 3.0 x 10-9 M DNZ at (a) bare CPE and (b) CDCMPE.
On scanning from the potential 0 to -1.5 V, the compound yields one peak, which is due to the reduction of the carbonyl group [20,21] and no peak is observed on the anodic branch, indicating that the reduction of donepezil is irreversible. For the reduction peaks observed, the voltammetric currents at CDMCPE are higher in comparison with the bare CPE, indicating that the CDMCPE has better efficiency for accumulating drugs. The variations in the voltammograms can be ascribed to the immobilization of the β cyclodextrin modified carbon paste electrode and the unmodified carbon paste electrode. It leads to a modification of the electrode surface causing a significant increase in the signal due to drug accumulation by the formation of an inclusion complex between β-cyclodextrin and carbonyl group, according to Dang et al. [22].
Differential Pulse Adsorptive Stripping Voltammetry
Fig. 3 illustrates DPV for 3.0x10-9 M donepezil with bare CPE and modified CDMCPE. The higher peak is observed at CDMCPE in comparison with bare CPE. The systematic studies of various experimental and instrumental parameters that affect the adsorptive stripping voltammogram response are carried out to establish the optimum conditions. From the obtained results, the following electrode mechanism can be proposed:
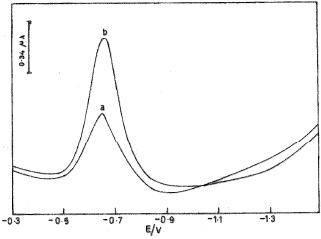
Figure 3. Typical DPV of 3.0 x 10-9 M DNZ at (a) bare CPE and (b) CDCMPE.
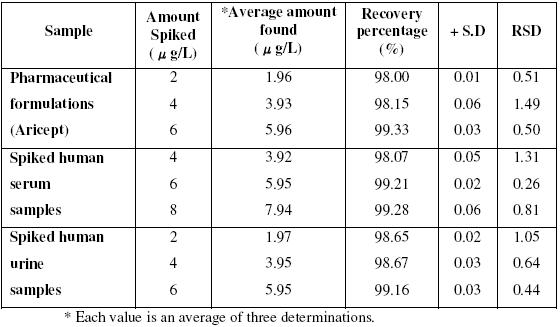
Determination of DNZ in pharmaceutical formulations by DPAdSV in BRB of pH 3.0 at CDMCPE
The DPV method is applied to the direct determination of donepezil in pharmaceutical formulations by using CDMCPE. The accuracy of the method using CDMCPE is determined by its recovery during spiked experiments. The standard addition procedure is employed and achieved successful results with proposed DPV technique and the obtained data are shown in table 3. The recoveries of donepezil from the pharmaceutical samples are satisfactory. These recoveries are compared with the mean recovery of donepezil discussed by C. Apostolou et al. [23]. The recovery values are achieved at CDMCPE which are better than that of the values obtained in the reference method. This means that the proposed technique should be applicable to the analysis of the above mentioned formulations and other similar type of formulation products containing donepezil with great success.
Table 3. Determination of DNZ in pharmaceutical formulations, human serum samples and human urine samples by DPAdSV in BRB of pH 3.0 at CDMCPE.
Determination of DNZ in spiked human serum samples by DPV in BRB of pH 3.0 at CDMCPE
Human serum samples are obtained from healthy individuals and are stored frozen until assay. An aliquot volume of serum sample is spiked with donepezil solution individually. 2x10-3 M concentrated drug solutions are diluted to 1 mL volume with acetonitrile in a 2.5 mL centrifuge tube; this is vortexed for 10 min and centrifuged for 5 min at 2000 rpm for removing of protein residues. The separated supernatant of the sample was taken carefully; from this sample required volume of solution is transferred into the electrolytic cell containing BR buffer of pH 3.0. The voltammograms are recorded by the differential pulse voltammetry method and the data are shown in the table 3.
Determination of DNZ in spiked human urine samples by DPV in BRB of pH 3.0 at CDMCPE
Blank urine samples are collected (for 24 hrs) from healthy male voluntaries. This is added to the 50 mL voltammetric cell containing BR buffer of pH 3.0. Firstly blank urine sample is introduced and voltammograms are recorded, and then spiked with donepezil. Each time, the differential pulse adsorptive stripping voltammetric signals are recorded and the data are given in the table 3. These recoveries are compared with the mean recovery of donepezil discussed by C. Apostolou et al. [23] and found better over the reference method.
Conclusions
The present method certainly is used as an alternative to the colorimetric, spectrophotometric and chromatographic methods. Electrochemical technique is easy to handle, cheaper and time saving. The determination in pharmaceutical formulations and spiked urine and blood serum samples without any preliminary treatment, by DPV with a CDMCPE is a suitable method. Further due to CDMCPE, stability, accuracy and low cost, it offers a good possibility as a substitute for the previous approaches used in routine analysis.
Acknowledgements
One of the authors, P. Venkateswarlu, is grateful to University Grants Commission, New Delhi, for financial assistance.
References
1. L. Istasse, Y. Yamanishi, Basic, Clinical, and Therapeutic Aspects of Alzheimers and Parkinsons Diseases, vol. 2, Plenum Press, New York, 1990, a. 409.
2. S.L. Rogers, R.S. Doody, R.C. Mohs et al., Arch. Intern. Med. 158 (1998) 1021-1031.
3. T.H.Y. Sugimoto, Y. Tsuchiya, H. Sugumi, K. Higurashi, N. Karibe, Y. Iimura, A. Sasaki, Y. Kawakami, T. Nakamura, J. Med. Chem.33 (1990) 1880-1887. [10.1021/jm00169a008]
4. S.L. Roger, L.T. Friedhoff, Br. J. Clin. Pharmacol. S46 (1998) 1-6.
5. S.L. Roger, M.N. Cooper, R. Sukovaty, et al., Br. J. Clin. Pharmacol. S46 (1998) 7-12.
6. P.J. Tiseo, C.A. Perdomo, L.T. Friedhoff, Br. J. Clin. Pharmacol. S46 (1998) 19-24.
7. P.J. Tiseo, R. Vargas, C.A. Perdomo, et al., Br. J. Clin. Pharmacol. S46 (1998) 51-55.
8. P.J. Tiseo, K. Foley, L.T. Friedhoff, Br. J. Clin. Pharmacol. S46 (1998) 56-60.
9. N.Y. Furukori, R. Furuya, T. Takahata, T. Tateishi, J. Chromatogr. B. 768 (2002) 261-265. [10.1016/S1570-0232(01)00592-X]
10. H. Pappa, R. Farru, P.O. Vilanova, M. Palacios, M.T. Pizzorno, J. Pharm. Biomed. Anal. 27 (2002) 177-182.
11. J. Haginaka, C. Seyama, J. Chromatogr. B 577 (1992) 95-102. [10.1016/0378-4347(92)80602-M]
12. K. Nakashima, K. Itoh, M. Kono, M.N. Nakashima, M. Wada, J. Pharm. Biomed. Anal. 41 (2006) 201-206. [10.1016/j.jpba.2005.10.017]
13. K. Matsui, Y. Oda, H. Ohe, S. Tanaka, N. Asakawa, J. Chromatogr. A 694 (1995) 209-218. [10.1016/0021-9673(94)01005-Y]
14. M.L. Bender, Komi yam, M. Cyclodextrin Chemistry; Springer verlag. New York, 1977.
15. J. Szejtti, Cyclodextrin Technology; Kulwer Academic publishers; Bosten, 1988.
16. C. T. Matsue, M. Fujihira, T. Osa, Bull. Chem. Soc. Jpn. 56 (1983) 1305-1307. [10.1246/bcsj.56.1305]
17. T.M. Reddy, M. Sreedhar, S.J. Reddy. Anal. Lett. 36 (2003) 1365-1379. [10.1081/AL-120021092]
18. A. Ferancova, J. Labuda, Fresenius J. Anal. Chem. 370 (2001) 1-10. [10.1007/s002160100752]
19. P.M. Bersier, J. Bersier, B. Klingert, Electroanalysis 3 (1991) 443-455. [10.1002/elan.1140030603]
20. J.E. Belgaied, Anal. Bio. Chem. 376 (2003) 706. [10.1007/s00216-003-1959-1]
21. C. Jeyaseelan, A.P. Joshi. Anal. Bio. Chem. 373 (2002) 772-776. [10.1007/s00216-002-1408-6]
22. L.G. McLaughlin, J.D. Henion, J. Chromatogr. B 529 (1990) 1. [10.1016/S0378-4347(00)83803-7]
23. C. Apostolou, Y. Dotsikas, C. Kousoulos, Y.L. Loukas, J. Chromatogr. B 848 (2007) 239-244. [10.1016/j.jchromb.2006.10.037]
24. G.F. Sales, C. Martins, M.F. Barrasso, M.C.V.F. Vaz, M.B.P. Oliveira, C. Delerue-Matos, Port. Electrochim. Acta 25 (2007) 173-183. [10.4152/pea.200701173] [ Links ]
25. Ahmad H. Alghamdi, Ali F. Alghamdi, Mohammed A. Alomar, Port. Electrochim. Acta 27 (2009) 645-655. [10.4152/pea.200906645] [ Links ]
26. M.F. Barrasso, T. Santos, M. Goreti F. Sales, M. Carmo V. F. Vaz, Port. Electrochim. Acta, 25 (2007) 119-129. [10.4152/pea.200701119] [ Links ]
27. I.H.I. Habib, S.A. Weshay, S. Toubar, M.M.A. Alamin, Port. Electrochim. Acta, 26 (2008) 315-324. [10.4152/pea.200804315] [ Links ]
28. C.N. Rao, M. Suman, C.N. Rao, P. Venkateswarlu, Mater. Sci. Research India 5 (2008) 383-390.
29. C.N. Rao, K. Balaji, C.N. Rao, P. Venkateswarlu, Mater. Sci. Research India 5 (2008) 305-312.
30. C.N. Rao, K. Balaji, C.N. Rao, P. Venkateswarlu, Biom. Pharmac. J. 2 (2009) 169-172.
31. C.N. Rao, K. Balaji, C.N. Rao, P. Venkateswarlu, Biom. Pharmac. J. 2 (2009) 117-121.
32. C.N. Rao, K. Balaji, C.N. Rao, P. Venkateswarlu, Current World Environment 4 (2009) 133-136.
Received 15 July 2010; accepted 25 November 2010
*Corresponding author
E-mail address: pvprofvenkat51@gmail.com














