Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portugaliae Electrochimica Acta
versão impressa ISSN 0872-1904
Port. Electrochim. Acta v.28 n.5 Coimbra 2010
Electrochemical Deposition and Characterization of Poly(3,4-ethylene dioxythiophene), Poly(aniline) and their Copolymer onto Glassy Carbon Electrodes for Potential Use in Ascorbic Acid Oxidation
G.M. El-Enany,1 M.A. Ghanem,2,* M.A. Abd El-Ghaffar3
1 Science and Maths Department, Faculty of Engineering, Port-Said University, Port Said, Egypt
2 Science and Maths Department, Faculty of Petroleum and Mining Engineering, Suez Canal University, Suez, Egypt
3 Polymers and Pigments Department, National Research Centre, Cairo, Egypt
DOI: 10.4152/pea.201005336
Abstract
Poly(3,4-ethylene dioxythiophene) (PEDOT), poly(aniline) (PANI) and their copolymer (PEDOT-PANI) were electrodeposited onto a glassy carbon electrode. The electrodeposition was performed using cyclic voltammetry from acidic solution containing appropriate monomer concentrations and sodium dodecyl sulphate (SDS) as a wetting agent. The resulting polymer films were characterised using cyclic voltammetry in acidic and neutral phosphate buffer solutions and IR spectroscopy. The specific capacitance of the PEDOT-PANI co-polymer reaches up to 260 F g−1 and had good stability during cycling in mineral acid solution. IR spectroscopy confirms the formation of PEDOT-PANI copolymer. The polymers showed an electrochemical activity towards ascorbic acid oxidation. The oxidation current was linearly dependant up to 20 mM ascorbic acid concentration and the PEDOT activity was much higher than that for PANI and PEDOT-PANI copolymer.
Keywords: poly(3,4-ethylene dioxythiophene),poly(aniline), cyclic voltammetry, electrochemical polymerization, electrochemical oxidation, ascorbic acid, specific capacitance.
Introduction
Conducting polymers such as poly(acetylene), poly(thiophene), poly(pyrrole) and poly(aniline) have attracted considerable interest over the last two decades because of their potential applications in different technologies such as electrochemical displays, smart windows, sensors, catalysis, capacitors, antistatic coatings and batteries [1-4]. The chemical structure of conducting polymers and the available methods of assembly make them compatible with many biological systems which lead to the application of conducting polymers in biosensors [5,6]. The key issues for the development of conducting polymer based electrochemical sensors and biosensors for environmental and biomedical monitoring are long-term stability, high sensitivity, short response time, good biological activity and compatibility. Another key factor is the mild conditions used for electrochemical polymerization which are ideal for incorporation of enzymes and antibody sensors [5,6]. The most widely studied conducting polymers in biosensors are poly(pyrrole) and poly(aniline). Simple poly(thiophene) and its derivatives require high oxidation potentials and these monomers are not water-soluble, which limits their applications in biosensors. Poly(3,4-ethylenedioxythiophene) (PEDOT), polyaniline (PANI) and their derivatives have the advantage of greater chemical stability among the organic conducting polymers [6-8]. PEDOT has linear chains which block the 3,4-positions of the thiophene ring. This causes the high degree of visible light transmission and high environmental stability in the conducting state, along with a tendency toward multiple redox switching due to its ease of oxidation [9]. The most popular methods to prepare PANI are chemical and electrochemical polymerization of aniline in an acidic medium [10]. Numerous reports have studied the redox behaviour of PANI coatings as well as the role of the dopant anions [10-15]. It has been reported that the addition of mineral acid in large quantities causes an enhancement in reactivity of the growing chains but may also generate undesirable reactive sites [16].
Copolymerization is often used to prepare a new polymer with properties that differ from the constituent homopolymers. Generally, the physical and chemical properties of the co-polymer are intermediate between those of the respective homopolymers but significantly distinct from those of the composite and blend. The copolymerizations of EDOT and other monomers such as 3-methylthiophene [17], N-ethylcarbazole [7], pyrrole and N-substituted carbazole [18] have been widely reported because of their unique properties.
Among co-polymer modified electrodes, PEDOT-PANI has received a great deal of attention as an electrode material because it exhibits some very interesting properties in light emitting devices [19-22] and in electrochemical catalysis [23]. The synthesis of these copolymers is difficult due to the difference in the oxidation potentials of the two monomers, i.e., the monomer with the lower oxidation potential is preferentially polymerized on the electrode surface. The quality of the deposited polymer on the electrode has been shown to be strongly dependent on the experimental conditions such as solvent, dipping time, electrode surface state [24], monomer structure [25] and concentration [26], all of them having profound influences on the structure and the electrochemical properties of the resulting polymers. Randriamahazaka et al. carried out electropolymerization of PEDOT and PANI in acetonitrile solution; they observed that the oxidized PEDOT film exhibited a Lewis acid behaviour that allowed the polymerization of poly(aniline) on PEDOT modified electrodes in the absence of protic acid [22].
Malinauskas et al. have given a short review on the electroanalytical aspects of ascorbic acid oxidation at conducting modified electrodes [27]. Ascorbic acid is an important analyte that is present in many biological fluids, juices, soft drinks, pharmaceutical formulations, etc. [27,28]. Various types of poly(aniline) modified electrode and techniques have been used for the electrooxidation of ascorbic acid [29-33].
Here we attempted to incorporate the EDOT with aniline monomer units through copolymerization to improve PANI properties through this particular combination. The PEDOT, PANI and their copolymer (PEDOT-PANI) were prepared by electrodeposition on glassy carbon electrodes. The polymer electrodeposition was performed using cyclic voltammetry from acidic solution containing appropriate concentrations of monomers and sodium dodecyl sulphate (SDS) as emulsifier. The resulting PEDOT, PANI and PEDOT-PANI polymer films were characterised by cyclic voltammetery and IR spectroscopy. The specific capacity and the electrocatalytic activities of the resulting polymers towards ascorbic acid oxidation were evaluated in phosphate buffer solution.
Experimental
The monomer 3,4-ethylenedioxythiophene EDOT was purchased from Bayer Company, and aniline from Aldrich. Sulphuric acid 98%, phosphoric acid, ascorbic acid (AA) and sodium dodecyl sulphate (SDS) were provided by El-Nasr Pharmaceutical Chemicals (ADWIC), Egypt. Deionized water (resistivity 18.0 M Ω cm) was used for the preparation of all solutions. The working electrode was made from a glassy carbon disk 3.0 mm diameter (SIGRADUR, Germany) by sealing 1.0 cm long x 3.0 mm diameter GC rod in glass tube and connected by copper wire using melted indium (Aldrich). Prior to each experiment the GC electrode was polished with alumina powder (1.0 and 0.5 μm) to a mirror finish, then sonicated in water to remove any residual alumina particles, dried in air and used immediately.
All electrochemical experiments were carried out using SP-150 Potentiostat/galvanostat (BioLogic) connected to a computer with EC-Lab software. The electrochemical cell was a conventional three electrodes system with a platinum mesh as the counter electrode and a saturated calomel electrode (SCE) as the reference electrode.
The aqueous solution used for the polymerization contained 0.5 M H2SO4 as supporting electrolyte and 70 mM SDS as wetting agent. The monomer concentration was 50 mM in case of single polymerization, while in the case of co-polymerization the concentration ratio was 1:1 at 25 mM for each monomer. Prior to each experiment the solution was purged with nitrogen gas for 20 min to remove the dissolved oxygen. Polymerization was carried out at glassy carbon electrodes by cycling in the potential range from -0.4 to 1.2 V vs. SCE at scan rate of 50 mV s−1. The resulting polymer films were characterized by cyclic voltammetry in 1.0 M H2SO4 or phosphate buffer solution (pH = 7.0). The polymer films used for IR characterisation were formed on flat ITO substrates by cycling between -0.4 to 1.2 V vs. SCE at 50 mV s−1. Infrared spectra in the range 400-4000 cm-1 were recorded using a Perkin-Elmer 1650 FTIR spectrophotometer.
Amperometric detection of ascorbic acid
To study the effect of ascorbate concentration using PEDOT and PANI and PEDOT-PANI co-polymer modified electrodes, a suitable sensing potential of 0.5 V was chosen in the limited current plateau region as determined by the cyclic voltammetry at 10 mV s-1 in a solution containing 0.1 M phosphate buffer solution (PBS) pH = 7.0 and 10 mM AA. The relation between concentration and limiting current was determined by adding different aliquots of AA in 0.1 M PBS to the background solution (pH adjusted back from 3.0 to 7.0 by titration using 5.0 M NaOH). The current was sampled after each step change in ascorbic acid concentration and the calibration curve was constructed. A thermostatic bath was used to fix the solution temperature at 25.0 ± 0.1 oC.
Results and discussion
Electrochemical polymerization of ANI, EDOT and EDOT–ANI
Fig. 1 (a, b and c) shows the electrodeposition of PANI, PEDOT and PEDOT–PANI polymer and co-polymer films, respectively, at a glassy carbon electrode using successive cycling between -0.4 to 1.2 V vs. SCE at 50 mV s−1. The polymerization was performed from a solution containing 0.5 M H2SO4, 70 mM SDS as surfactant and 50 mM of the monomer. In the case of EDOT and EDOT-aniline binary mixtures, the electrocatalytic effect of SDS was characterized by a significant decrease of the EDOT oxidation potential in the micellar medium relative to acetonitrile as well as aqueous solutions. This behaviour might result from strong electrostatic interactions occurring between the EDOT●+ radical cations and the dodecylsulfate anions during the polymerization process [34]. From the data we can see that all CVs show redox peaks that are characteristic for polymer formation [1] and the current increases with each consecutive cycling, which indicates an increase in the amount of deposited polymer with each cycle. At the same time homogeneous and adherent polymer films were deposited on the anode surface, which can be observed by the naked eye.
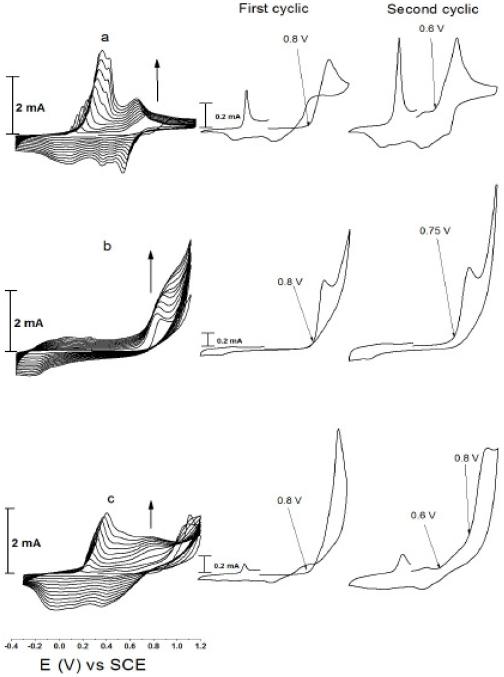
Figure 1. Cyclic voltammograms recorded at 50 mV s-1 for (a) 50 mM aniline, (b) 50 mM EDOT and (c) 25 mM aniline + 25 mM EDOT, in 70 mM SDS and 0.5 M H2SO4.
In Fig. 1(a) the onset of the polymerization potential of PANI shifts from 0.8 V on the first cycle to 0.6 V on the second cycle, indicating that the PANI formed during first cycle catalyses the formation of PANI on its surface. In Fig.1(b) and during the first scan, the polymerization potential of PEDOT on GC is 0.82 V, while on following scans the onset of polymerization shifts significantly to less positive potentials and stabilises at 0.75 V. In the case of aniline and EDOT binary mixture in Fig. 1(c) we chose aniline to EDOT concentration ratio to be 1:1 (25 mM for each momomer) and cycle between -0.4 and 1.2 V vs. SCE. In this case we expected some deactivation will occur during the formation of PANI due to overoxidation [11].
From the CV in Fig. 1(c) the behaviour is very similar to polymerization of aniline in the potential range 0.2 to 0.5 V, indicating that the polymerization of ANI proceeds first followed by EDOT polymerization, which starts to polymerize at potentials beyond 0.8 V.
The ratio between the partial polymerization current for PANI (at 0.8 V) to the partial polymerization current for PEDOT (at 1.2 V) was 1:1 in each cycle. This can be related to the formation of co-polymer which may be formed at potentials more positive than 0.8 V whereas below 0.8 V only PANI is formed. This indicates that the film formed from the EDOT and aniline binary mixture consisted of a blend of PANI and PEDOT-co-PANI polymers, as shown in Scheme 1.

Scheme 1.
The electrodeposited PEDOT, PANI and PEDOT–PANI co-polymers were examined by IR spectroscopy. Fig. 2(a) shows the IR spectrum for PEDOT film prepared by cycling from -0.4 to 1.2 V vs. SCE at 50 mV s−1. The spectrum shows several bands of which the band at 819 cm-1 is assigned to the symmetric C-S-C deformation [35], and the band at 1053.91 cm-1 is assigned to the symmetric C─O─C ether bond. In the case of PANI, as shown in Fig. 2(b), there are two characteristic bands at 1595.8 cm-1 assigned to the C=N, and 1285 cm-1 assigned to the C-N [36]. In the case of the PEDOT-PANI co-polymer the IR spectrum in Fig. 2(c) shows the band at 1056 cm-1 which is assigned to C─O─C ether bond of PEDOT and the band at 1285 cm-1 is assigned to the C─N bond in PANI. The existence of these two bands confirms the formation of PEDOT-PANI co-polymer.
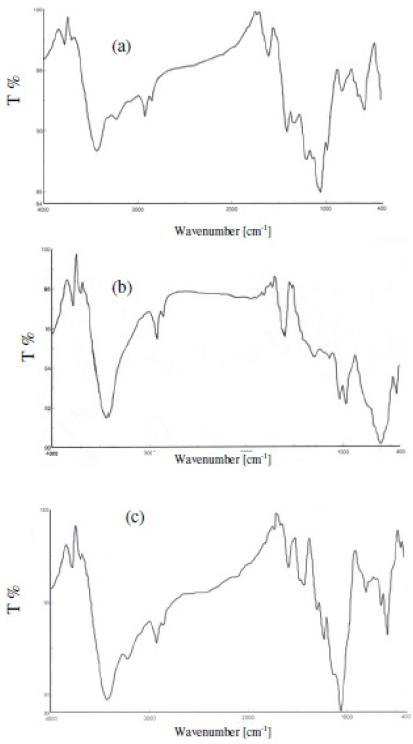
Figure 2. IR spectra for a) PEDOT, b) PANI and c) PEDOT-PANI.
Electrochemistry of as-formed PEDOT, PANI and PEDOT–PANI films
The electrochemical behavior of the PEDOT films deposited electrochemically from aqueous solution containing surfactants was studied in 1.0 M H2SO4 aqueous solution. These films can be cycled repeatedly between the conducting (oxidized) and insulating (neutral) state without significant decomposition of the material, which is consistent with the results reported in the literature [37].
The PEDOT, PANI and PEDOT-PANI polymer films coated on the working electrode are cycled between oxidized and neutral (doping de-doping) states at various scan rates in 1.0 M H2SO4 aqueous solution. For the electroactive species that are confined at the electrode surface, both the anodic (Ia) and cathodic current (Ic) responses increase linearly with the scan rate [24]. As seen from Fig. 3(a) and in H2SO4 solution the anodic peak current values are linearly dependant on the square root of the scan rate, which indicates that the doping de-doping process is controlled by diffusion in a consistent way with reports in the literature [38].
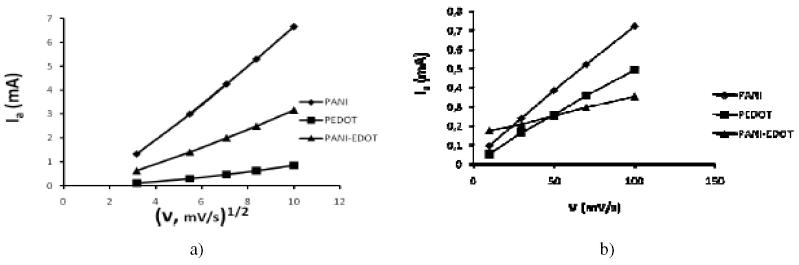
Figure 3. a) Anodic peak currents (Ia) as a function of the square root of the scan rate for PEDOT, PANI and PANI-PEDOT polymer films in 1.0 M H2SO4 solution. b) Relationship between the anodic (Ia) peak currents and the scan rate for PEDOT, PANI and PANI-PEDOT polymer films in PBS (pH = 7.0).
On the other hand, in the case of cycling the polymer films in PBS (pH = 7.0) the anodic peak current increases linearly with increasing scan rate, as shown in Fig. 3(b), indicating that the redox process is non-diffusional and that the electroactive polymer is well adhered to the surface of the glassy carbon electrode. Possibly in PBS the doping de-doping process only takes place at the outer polymer surface, and more investigation needs to be carried out using rotating disk electrodes for different film thickness to fully understand this behaviour.
PEDOT-PANI co-polymer electrode was prepared by electropolymerization of a binary-monomer mixture of aniline and EDOT to obtain the PEDOT-PANI co-polymer, which shows higher specific capacitance, being economically cheap and easily prepared.
The specific capacitance of the PEDOT, PAN and PEDOT-PANI co-polymer electrodes can be estimated from equation (1):
![]()
where C is the specific capacitance (F g-1), Q is the voltammetric charge (C), ΔE is the potential window, and m is the mass of the polymer material (g) estimated from the polymerization charge suggesting the polymerisation efficiency was 100% [39].
The specific capacitance of PEDOT-PANI co-polymer electrodes was much higher than the values for either pure PEDOT or pure PANI electrodes due to the synergistic effect of PEDOT and PANI when using PBS (pH = 7.0) as the electrolyte (Fig. 4). Moreover, the specific capacitance of the PEDOT-PANI co-polymer reaches more than 170 F g−1 and showed good stability. This implies that the PEDOT-PANI co-polymer is a promising electrode material for supercapacitors [40]. In 1.0 M H2SO4, the specific capacitance of the PEDOT-PANI co-polymer electrode reaches more than (260 Fg-1), which is much higher than for the PEDOT electrode (55 Fg-1) and indicates that the presence of PANI enhances the specific capacitance of PEDOT. In addition, the fact that the specific capacity of the PEDOT-PANI co-polymer electrode in PBS buffer is less than in 1.0 M H2SO4 may be attributed to the lower activity of PANI in neutral solution, predominating the PEDOT behavior.
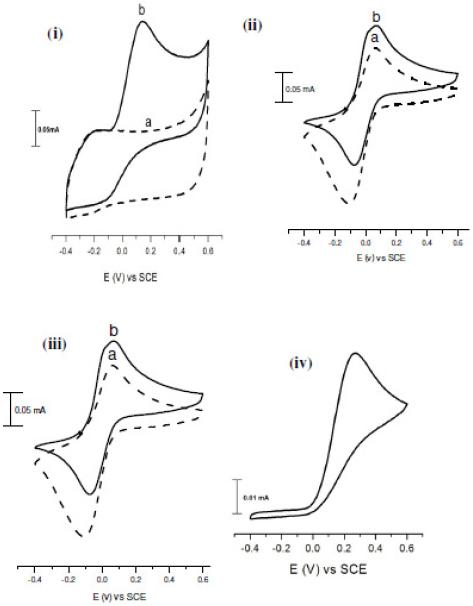
Figure 4. Cyclic voltammograms recorded at 10 mV s-1 for polymer films in (a) phosphate buffer solution (pH = 7.0) and (b) in the same solution after adding 10 mM ascorbic acid: (i) PEDOT, (ii) PANI, (iii) PANI-PEDOT co-polymer and (iv) polished GC electrode.
Electrochemical response of ascorbic acid at PEDOT, PANI, and PEDOT–PANI modified GC electrodes
Fig. 4 illustrates the electrocatalytic behaviour of the (i) PEDOT, (ii) PANI and (iii) PANI-PEDOT co-polymer in PBS (pH = 7.0) containing 10 mM of ascorbic acid. The cyclic voltammetry at polished GC electrodes (iv) also was tested in the same solution for comparison. The cyclic voltammograms of ascorbate anion in the PBS produced a single irreversible oxidation peak at both modified and polished electrode surfaces. The reason for this phenomenon is the reaction of the oxidized form of ascorbic acid with water that results in irreversible electrochemical behaviour [41]. The peak potential for oxidation of ascorbate anion occurred at around 0.30 V at the polished GC electrode, while it occurs at about 0.10 V for PEDOT, PANI and PEDOT-PANI modified GC electrodes. The oxidation potential of ascorbic acid at these modified polymer electrodes clearly shifted about 200 mV towards less positive potentials with a large increase in the peak catalytic current compared to that at the polished GC electrode. This negative potential shift can be attributed to the mediation (catalytic) effect of the polymers on the ascorbate oxidation reaction in neutral solution [42]. In addition, PEDOT modified electrode shows a bigger increase in oxidation current than the PANI and PEDOT-PANI copolymer, which can be interpreted as being due to the preconcentration of the negatively charged ascorbate anion in the PEDOT matrix because of the electrostatic attraction with the positively charged PEDOT chains in the oxidized state. [43].
Fig. 5 shows the amperometric response at 0.5 V vs. SCE for the PEDOT, PANI and PEDOT-PANI co-polymer electrodes to the consecutive additions of ascorbic acid. The transients were obtained by adding different aliquots of ascorbic acid in 0.1 M PBS to the background solution and the current was sampled after each step change in ascorbic acid concentration. From Fig. 5 we note that the current only starts to increase on the addition of 2.0 µM ascorbate in the case of the PEDOT electrode, while it starts to increase after addition of 0.1 mM and 0.3 mM ascorbate in the cases of the PEDOT-PANI co-polymer and PANI electrodes, respectively. This reveals the higher sensitivity of PEDOT toward ascorbate oxidation. The polymers showed electro-catalytic activity towards ascorbate oxidation and the oxidation current was linearly dependant on ascorbate concentrations above 1.0 mM.
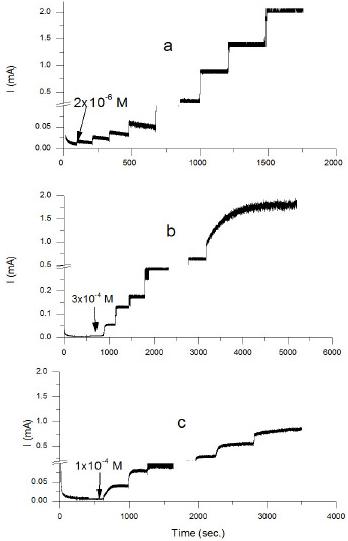
Figure 5. Current time transients recorded at 0.5 V vs. SCE for (a) PEDOT, (b) PANI and (c) PEDOT-PANI co-polymer electrodes after successive additions of ascorbic acid. The polymer was formed by electrochemical polymerization by cycling between -0.40 and 1.20 V in 70 mM SDS, 0.5 M H2SO4 and 50 mM monomer at 50 mV s-1.
The PEDOT activity was much higher than that for PANI and PEDOT-PANI, as shown in Fig. 6. The higher sensitivity of PEDOT toward ascorbate oxidation (oxidation current 0.985 mA mmol cm-2) can be attributed to the electrostatic interaction between the ascorbate anions and the positively charged groups of the polymer film [44,45]. Atta et al. [46] reported that the hetero atom present in the polythiophene film was involved in electron transfer reactions. They explained this behaviour by the masking of the active of hetero-atom sites by molybdenum ions and demonstrated the decrease in the catalytic effect at the polymer film.
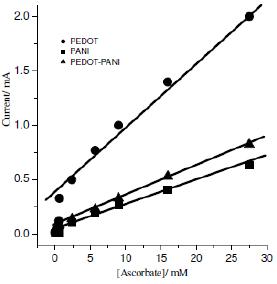
Figure 6. Relationship between the ascorbic acid oxidation current and concentration for the (●) PEDOT, (■) PANI polymer and (▲) PEDOT-PANI co-polymer.
Conclusions
In summary PEDOT and PANI polymers, and PEDOT-PANI co-polymers were electrodeposited onto the surface of glassy carbon electrodes by cyclic voltammetry from aqueous miceller solution. The electrochemical and electrocatalytic properties of the resulting polymer films were examined in H2SO4 and in phosphate buffer solutions (pH = 7.0). The specific capacity of PEDOT was enhanced by simultaneous co-polymerization of EDOT and aniline, to reach up to 260 F g-1, which was much higher than that for the PEDOT electrode (55 F g-1) and had good stability. This implies that PEDOT-PANI co-polymer is a promising electrode material for supercapacitors. The polymers showed electro-catalytic activity towards ascorbate oxidation and the oxidation current was linearly dependant on ascorbic acid concentration up to 20.0 mM.
The PEDOT activity was much higher than that for PANI and PEDOT-PANI and the oxidation current sensitivity reached 0.985 mA mmol cm-2.
Acknowledgment
The authors thank Prof. Philip Bartlett at University of Southampton, UK, for the proof reading and revising this work.
References
1. T.A. Skotheim, R.L. Elsenbaumer, J.R. Reynolds, Handbook of Conducting Polymers, Marcel Dekker, New York, 1998
2. J.D. Stenger-Smith, C.K. Webber, N. Anderson, A.P. Chafin, K. Zong, J.R. Reynolds, J. Electrochem. Soc. 149 (2002) A973. [10.1149/1.1485773]
3. H. Randriamahazaka, V. Nöel, C. Chevrot, J. Electroanal. Chem. 521 (2002) 107. [10.1016/S0022-0728(02)00665-4]
4. G. Inzelt, M. Piner, J.W. Schultze, M.A. Vorotyntsev, Electrochim. Acta 45 (2000) 2403. [10.1016/S0013-4686(00)00329-7] [ Links ]
5. S. Cosnier, Biosens. Bioelectron. 14 (1999) 443. [10.1016/S0956-5663(99)00024-X]
6. G.G. Wallace, M. Smyth, H. Zhao, Trends Anal. Chem. 18 (1999) 245. [10.1016/S0165-9936(98)00113-7]
7. M.A. del Valle, P. Cury, R. Schrebler, Electrochim. Acta 48 (2002) 397. [10.1016/S0013-4686(02)00685-0] [ Links ]
8. M. Dietrich, J. Heinze, G. Heywang, F. Jonas, J. Electroanal. Chem. 369 (1994) 87. [10.1016/0022-0728(94)87085-3]
9. S. Sadki, C. Chevrot, Electrochim. Acta 48 (2003) 733. [10.1016/S0013-4686(02)00742-9] [ Links ]
10. S. Palaniappan, Eur. Polym. J. 37 (2001) 975. [10.1016/S0014-3057(00)00207-X]
11. F. Tran-Van, S. Garrean, G. Louarn, G. Froyer, C. Chevrot, J. Mater. Chem. 11 (2001) 1378. [10.1039/b100033k]
12. A. Eftekhari, P. Jafarkhani, Polym. J. 38 (2006) 651. [10.1295/polymj.PJ2005181]
13. E. Munoz, A. Colina, A. Heras, V. Ruiz, S. Palmero, J. López-Palacios, Anal. Chim. Acta 573/574 (2006) 20. [10.1016/j.aca.2006.01.029]
14. S.Y. Hong, Y.M. Jung, S. Bin Kim, S.M. Park, J. Phys. Chem. B 109 (2005) 3844. [10.1021/jp046218f]
15. C. Dalmolin, S.C. Canobre, S.R. Biaggio, R.C. Rocha-Filho, N. Bocchi, J. Electroanal. Chem. 578 (2005) 9. [10.1016/j.jelechem.2004.12.011]
16. G.A. Ragoisha, A.S. Bondarenko, Electrochim. Acta 50 (2005) 1553. [10.1016/j.electacta.2004.10.055] [ Links ]
17. N. Gospodinova, L. Terlemezyan, Prog. Polym. Sci. 23 (1998) 1443. [10.1016/S0079-6700(98)00008-2]
18. T. Yohannes, J.C. Carlberg, O. Inganas, T. Solomon, Synth. Met. 88 (1997) 15. [10.1016/S0379-6779(97)80878-8]
19. J.H. Kang, Y.J. Oh, S.M. Paek, S.J. Hwang, J.H. Choy, Solar Energy Mater. Solar Cell 93 (2009) 2040. [10.1016/j.solmat.2009.08.007]
20. T.H. Lin, K.C. Ho, Solar Energy Mater. Solar Cell 90 (2006) 506. [10.1016/j.solmat.2005.02.017]
21. D. DeLongchamp, P.T. Hammond, Adv. Mater. 13 (2001) 1455. [10.1002/1521-4095(200110)13:19<1452::AID-ADMA1452>3.0.CO;2-P]
22. H. Randriamahazaka, V. Noël, S. Guillerez, C. Chevrot, J. Electroanal. Chem. 585 (2005) 157. [10.1016/j.jelechem.2005.05.015]
23. L. Zhang, H. Peng, P.A. Kilmartin, C. Soeller, J. T. Sejdic, Macromolecules 41 (2008) 7671. [10.1021/ma8013228]
24. Z. Mekhalif, P. Lang, F. Garnier, J. Electroanal. Chem. 399 (1995) 61. [10.1016/0022-0728(95)04172-9]
25. L.L. Davidson, C.V. Pham, H. Zimmer, H.B. Mark, D.J. Ondrus, J. Electrochem. Soc. 135 (1988) 1406. [10.1149/1.2096007]
26. J.R. Reynolds, S.G. Hsu, J. Polym. Sci., Part B: Polym. Phys. 27 (1989) 2081. [10.1002/polb.1989.090271012]
27. A. Malinauskas, R. Garjonyte, R. Mazeikiene, I. Jureviciute, Talanta 64 (2004) 121. [10.1016/j.talanta.2004.02.010]
28. S.P. Arya, M. Mahajan, P. Jain, Anal. Sci. 14 (1998) 889. [10.2116/analsci.14.889]
29. S.P. Arya, M. Mahajan, P. Jain, Anal. Chim. Acta 417 (2000) 1. [10.1016/S0003-2670(00)00909-0]
30. S.L. Mu, J.Q. Kan, Synth. Met. 132 (2002) 29. [10.1016/S0379-6779(02)00209-6]
31. R.P. Kalakodimi, M. Nookala, Anal. Chem. 74 (2002) 5531. [10.1021/ac025938k]
32. R. MaZeikiene, G. Niaura, A. Malinauskas, Synth. Met. 139 (2003) 89. [10.1016/S0379-6779(03)00037-7]
33. D.M. Zhou, J.J. Xu, H.Y. Chen, H.Q. Fang, Electroanalysis 9 (1997) 1185. [10.1002/elan.1140091512]
34. J.J. Xu, D.M. Zhou, H.Y. Chen, Fresenius J. Anal. Chem. 362 (1998) 234. [10.1007/s002160051066]
35. G. Lu, C. Li, J. Shen, Z. Chen, G. Shi, J. Phys. Chem. C 111 (2007) 5926. [10.1021/jp070387t]
36. M. Ibrahima, E. Koglin, Acta Chim. Slov. 52 (2005) 159.
37. X. Du, Z. Wang, Electrochim. Acta 48 (2003) 1713. [10.1016/S0013-4686(02)00011-7] [ Links ]
38. E.M. Genies, C. Tsintavis, J. Electroanal. Chem. 200 (1986) 127. [10.1016/0022-0728(86)90051-3]
39. F.J. Liu, J. Power Sources 182 (2008) 383. [10.1016/j.jpowsour.2008.04.008]
40. F. Joans, W. Krafft, B. Muys, Macromol. Symp. 100 (1995) 169.
41. K. Miyazaki, G. Mastsumoto, M. Yamada, S. Yasui, H. Kaneko, Electrochim. Acta 44 (1999) 3809. [10.1016/S0013-4686(99)00087-0]
42. P.N. Bartlett, E.N.K. Wallace, Phys. Chem. Chem. Phys. 3 (2001) 1491. [10.1039/B009377G]
43. S.S. Kumar, J. Mathiyarasu, K.L. Phani, Y.K. Jain, V. Yegnaraman, Electroanalysis 17 (2005) 2281. [10.1002/elan.200503375]
44. R.A. Saraceno, J.G. Pack, A.G. Ewing, J. Electroanal. Chem. 197 (1986) 265. [10.1016/0022-0728(86)80154-1]
45. P.R. Roy, T. Okajima, T. Ohsaka, Bioelectrochemistry 59 (2003) 11. [10.1016/S1567-5394(02)00156-1]
46. F. Atta, I. Marawi, K.L. Petticrew, H. Zimmer, H.B. Mark, A. Galal, J. Electroanal. Chem. 408 (1998) 47. [10.1016/0022-0728(95)04286-5]
Received 14 June 2010; accepted 23 November 2010
*Corresponding author
E-mail address: magomar67@yahoo.co.uk














