Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portugaliae Electrochimica Acta
versão impressa ISSN 0872-1904
Port. Electrochim. Acta v.28 n.5 Coimbra 2010
Influence of Chlorides, Nitrate and Sulphate Media on Corrosion Behaviour of TiO2 Particulate Reinforced Al-6061 Composites
H. C. Ananda Murthy,1 P. F. Sanaulla,2 V. Bheema Raju3,*
1 Department of Chemistry, R.N. Shetty Institute of Technology, Bangalore -560061, India
2 Department of Chemistry, HKBK College of Engineering, Bangalore -560045, India
3 Department of Chemistry, Dr. Ambedkar Institute of Technology, Visvesvaraya Technological Universitym Bangalore -560056, Karnataka, India
DOI: 10.4152/pea.201005309
Abstract
The corrosion behaviour of Al 6061-TiO2 particulate composites in chloride, nitrate, sulphate and acidic media has been studied in the present investigation. The unreinforced matrix, and the composites containing 2, 4 and 6 weight percent of TiO2 particulates were prepared by liquid metallurgy route using the vortex technique. Their corrosion behaviour was evaluated by weight loss, potentiodynamic polarisation, scanning electron micrograpaph (SEM) and energy dispersive X-ray analysis (EDXA) methods. The studies show that corrosion current density (ICorr) and the corrosion rate were highest in the case of corrodent 0.1 N HCl. The rate of corrosion of both the matrix alloy and the composites decreased with increase in time of exposure in acid medium, possibly due to the formation of a passive oxide layer. Corrosion current (ICorr), corrosion potential (ECorr) and pitting potential (EPit) were obtained from potentiodynamic polarisation studies. In all the media, the ICorr values of unreinforced matrix alloy and composites were found to increase as the TiO2 content increased from 0% to 6%, which could be attributed to the microgalvanic coupling between the reinforcement or conducting interfacial products and the matrix alloy. Insulator TiO2 particulates are perceived to act as inert material and degrade the integrity of the protective oxide layer on the Al matrix alloy. The presence of reinforcement in the matrix increases cathode to anode ratio in the composites, resulting in the formation of pits during localised corrosion. Moreover, the pitting effect was more pronounced in the presence of aggressive Cl- ions compared to mild NO3- and SO42- ions. SEM and EDX analysis of samples in chloride media showed the presence of large pits and completely deteriorated surface, complementing weight loss and polarisation studies.
Keywords: Al6061 MMCs, TiO2 particulates, SEM, EDXS, interfacial products.
Introduction
Particulate reinforced Al Metal Matrix Composites (PMMCs) have significant applications in various sectors such as structural avionics, automobiles, transport, nuclear and electronics industries. They are used as good substitutes for aluminium in automotive pistons, brakes, brake-drums, clutch discs, etc., owing to their better properties. Al alloys reinforced with ceramic oxides, carbides, nitrides and mineral silicate particulates are known for their attractive characteristics such as high specific modulus, high specific strength, low thermal expansion coefficient, light weight and low cost [1-3]. Al-6061 matrix has been an attractive matrix alloy for particulate reinforced composites and offers enormous prospects in space and avionic sectors. TiO2 having high hardness and modulus is a good reinforcement for aluminium matrix.
Corrosion studies published on Al-PMMCs in various salt media revealed significant variation in the corrosion resistance of these composites. Aluminium alloys reinforced with SiC [4], B [5], Al2O3 [6], TiC [7], and ZrB2 [8] are reported to show lower corrosion resistance for the composites compared to matrix alloys, owing to galvanic corrosion. However, garnet [9], albite [10], quartz [11] and glass fibre [12] reinforced Al composites exhibited higher corrosion resistance compared to their matrix alloys. The observed variation in resistance to corrosion in Al –MMCs is attributed to chemical or mechanical factors such as alloying segregation, interfacial reactions, oxidized layers, residual stress around reinforced particles in the matrix [13] and galvanic coupling between matrix and reinforcement [5]. The corrosion behaviour of reinforced Al composites has been earlier investigated [4-18] in acidic, neutral, alkaline and various salt media. Studies reveal that Al composites suffer greater localized pitting corrosion in chloride ion environment compared to other media [14-16].
The present investigation involves the study of corrosion behaviour of unreinforced Al6061 matrix and TiO2 particulate reinforced composites in different electrolytes like NaCl, NaNO3, Na2SO4 and HCl. The investigation was conducted using potentio-dynamic polarization technique and weight-loss method. Scanning electron micrograph (SEM) and energy dispersive X-ray (EDX) examinations of the electrode surface were also performed. TiO2 particulate reinforced Al-6061 composites exhibit nominal decrease in corrosion resistance when compared to matrix alloy.
Experimental
Materials selection
Aluminium alloy 6061-matrix
Aluminium alloy 6061 has the composition: Si – 0.6%, Fe - 0.1%, Cu – 0.3%, Mn – 0.02%, Mg – 0.8%, Zn, Cr, Ti – 0.01% each and remaining Al.
Reinforcement
TiO2, A.R Grade was obtained from E. Merck and used as reinforcement in the form of particulates.
Composite preparation
The liquid metallurgy route using the vortex technique was employed to prepare the composites. The composites were prepared containing 2, 4, and 6 percentage by weight of TiO2. Addition of reinforcement material TiO2, into the molten Al-6061 alloy (800 ºC) was carried out by creating a vortex in the melt using a mechanical stainless steel stirrer coated with alumina (to prevent migration of ferrous ions from the stirrer material to the alloy). The stirrer was rotated at a speed of 450 rpm in order to create the necessary vortex. The TiO2 particles were pre-heated to 400 oC and added into the vortex of liquid melt at a rate of 120 g/min. The TiO2 particulates were of size ~100 nm. The composite melt was thoroughly stirred and subsequently degasifiers were added. Castings were produced in permanent moulds in the form of cylindrical rods. [Diameter 30 mm and length 150 mm].
Specimen preparation
Cast material was cut into 20 x 20 mm cylindrical pieces using an abrasive cutting wheel. The matrix alloy was also cast under identical conditions as the composites, for comparison. The samples were successively ground using 240, 320, 400, 600, 800, 1000, 1500 and 2000 grade emery papers, were polished according to standard metallographic techniques, degreased in acetone and dried. The samples were weighed up to fourth decimal place using an electronic balance and the exact specimen dimensions were noted down. For polarisation studies, the samples were cut as cylindrical rods, welded with brass wire for electrical connection and moulded using acrylic rubber to offer an active flat disc shaped surface of 1 cm2. These working electrodes were also polished as described above.
Corrosion test: weight loss method
The corrosion behaviour of Al-6061 alloy was studied by static immersion test to measure the material loss. Weight loss measurements were carried out by weighing cylindrical specimens of pure Al6061 alloy and the composites, before and after immersion in 100 mL solutions of 0.1 N HCl. Samples were suspended in the corrosive medium for different time intervals up to 72 hours in steps of 24 h. After the specified time, the heavy corrosion deposits on the surface of samples were removed mechanically, then the samples were cleaned with distilled water, rinsed with acetone, dried and weighed. At least two samples were tested and the average value of the weight loss was determined. Corrosion rates were computed using the equation given by
Corrosion rate = 534 W / DAT mpy
where W is the weight loss in mg, D is the density of the specimen in g/cm3, A is the area of the specimen in sq-inch and T is the exposure time in hours.
Corrosion test: potentiodynamic polarisation method
Polarisation measurements were carried out on a Model 600 C series, Electrochemical Analyzer / Workstation, CH Instruments, USA. All Experiments were carried out using a three electrode cell with saturated calomel electrode (SCE) as reference, platinum electrode as counter electrode and the cylindrical specimens of the alloy with active flat disc of 1 cm2 as the working electrode. The SCE was connected via Luggin capillary, the tip of which was held very close to the surface of the working electrode to minimize the IR drop. Open Circuit Potential (OCP) measurements were recorded for 60 minutes, the time necessary to reach quasi stationary state for open circuit potential, followed by polarisation measurements at a scan rate of 1 mV/s for Tafel plots.
Energy dispersive X-ray (EDX) and scanning electron microscopy (SEM) analysis
To gain more insight on the composition of the corrosion products formed at the tested Al matrix and composites, the corroded samples from the polarization studies were subjected to energy dispersive x-ray analysis using model Oxford Link ISIS, UK. The morphology of the passive layer and the corrosion products formed on the electrode surface were examined by scanning electron micrograph (SEM) using model JSM-840A SEM, JEOL, Japan.
Results and discussion
The variation of corrosion rate with exposure time, for Al 6061 matrix alloy and its composites of TiO2 (2, 4 and 6%) in 0.1 N HCl medium, as obtained from weight loss method, is illustrated in Fig. 1. It can be observed that the corrosion rate for both matrix alloy and the composites decreases with increase in exposure time. This can be attributed to formation of Al hydroxide layer on the surface of the alloy and composites. Further, it can be observed that corrosion resistance of reinforced Al composites is lower than that of the matrix alloy, and the corrosion rate increases with increase in the weight percentage of TiO2 in the composites. The observed results can be explained as possibly due to a microgalvanic coupling between the products formed at TiO2 – matrix interface and the matrix alloy, although the TiO2 particles as such act as an inert material in the composites and may not influence the corrosion mechanism of the composites[12].
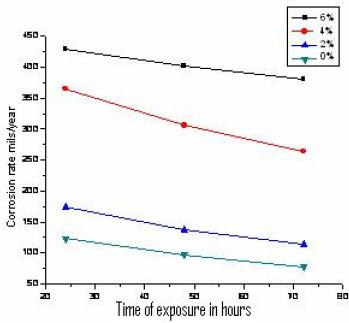
Figure 1. Variation of the corrosion rate for Al matrix alloy and its composites, with duration of immersion.
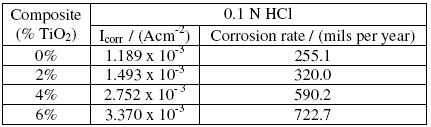
The results of weight loss method were complemented by those from potentiodynamic polarization studies. The electrochemical corrosion parameters of Al 6061 and its composites in 0.1 N HCl are given in Table 1. The corrosion current density Icorr, increases with increase of TiO2 content in the composites, corroborating the results that reinforcement with TiO2 does influence and increase the corrosion rate of the matrix alloy.
Table 1. Electrochemical corrosion parameters of Al6061 and its TiO2 composites in 0.1 N HCl solution.
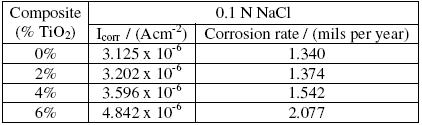
Typical polarisation curves for Al6061 matrix alloy and its TiO2 composites, in decinormal solution of NaCl, are shown in Fig. 2. It can be seen from the figure that all the curves and hence the polarization behaviour of Al6061 matrix alloy and its composites are similar. Similar plots are obtained, for the matrix and the composites, in 0.1 N NaNO3 and Na2SO4 media as well (hence not reproduced here). The evaluated electrochemical corrosion parameters for the matrix alloy and the composites in 0.1N NaCl are given in Table 2. It can be observed from the Tafel plots (Fig. 2) and Table 2 that the corrosion current (Icorr) values and the corrosion rate increase with increase in TiO2 content in the composites. These results point out to the fact that TiO2 reinforced composites have lower corrosion resistance as compared to matrix alloy in chloride medium. This observation is similar to the findings of Bhat et al. [4] in Al-SiC composites, Trzaskoma et al. in Al-Al2O3 composites [6] and Albiter et al. in Al-TiC composites[7], where the composites exhibit reduced corrosion resistance, compared to the matrix alloy. Further, comparing the Icorr values of the matrix alloy and the composites in HCl and NaCl media, from Tables 1 and 2, it can be observed that the corrosion rate is significantly higher in acidic chloride medium, compared to neutral salt chloride medium. Further, pitting of the samples was observed in both the media.
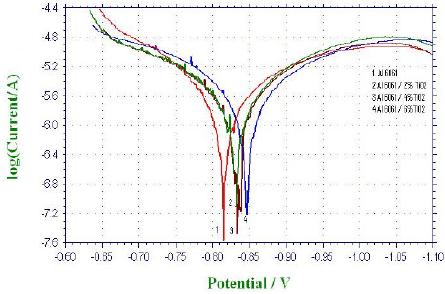
Figure 2. Tafel plots of Al6061 matrix (0%) and its TiO2 composites (2%, 4%, 6%) in 0.1 N NaCl medium.
Table 2. Electrochemical corrosion parameters of Al6061 and its TiO2 composites in 0.1 N NaCl solution.
It is anticipated that for obtaining good corrosion resistance, the potentials of the composite should shift to the more positive side as a function of time in any environmental conditions. There is no significant variation in corrosion potential (Ecorr) (from Fig. 2) between the matrix alloy and the composites, viz., –799 mV for matrix alloy to -822 mV for different TiO2 contents in composites. However, there is a small shift of corrosion potential values to the negative side in the composites, which is indicative of defect oxide layer formation in the presence of reinforcement in the matrix alloy [26].
Corrosion resistance of composites depends on many factors such as alloy composition, microstructure of matrix, techniques of preparation, porosity, precipitation of intermetallics, high dislocation densities at the particle-matrix interface, the presence of interfacial reaction products and insulating reinforcements [7, 24, 25].
In MMCs reinforced with conducting materials, the presence of more conductive phase at the interface is quoted [25] as providing an easier path for electron exchange for oxygen reduction and driving the anodic reaction at a higher rate. In Al6061-TiO2 composites, since the reinforcement TiO2 is an insulator, galvanic corrosion between the matrix - reinforcement may not be possible in composites [28]. However the formation of conducting intermetallic precipitates such as, Mg2Si and AlTi3 during fabrication of the composites could be the reason for increase in corrosion rates in composites [19]. Since aluminum is noble to Mg2Si, the microgalvanic coupling between them in Al6061 alloy and its composites would selectively corrode Mg2Si away [30].
The anodic polarization curves showing the pitting potentials of Al 6061 matrix alloy and its TiO2 composites in 0.1 N NaCl medium are shown in Fig. 3 and the corresponding pitting potentials Epit values are presented in the Table 3. The preferential corrosion starts at the interface, i.e. surrounding the TiO2 leading to a pitting type of corrosion. The presence of reinforcement in matrix acting as cathodic region increases with increasing TiO2 content and increases the corresponding cathode to anode ratio in composites, resulting in the formation of pits during localised corrosion. No significant change in pitting potential is observed [Fig. 3] in composites even though the addition of TiO2 reinforcement to Al6061 matrix alloy increases heterogeneity in the composites enhancing corrosion sensitivity in composites.
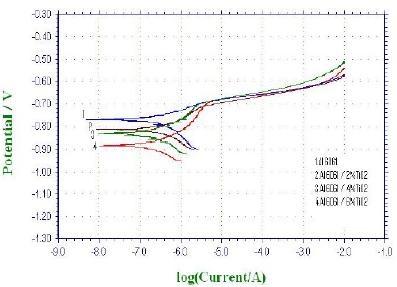
Figure 3. Anodic polarisation curves for composites containing 2, 4 and 6% by weight of TiO2 particulates in 0.1 N NaCl.
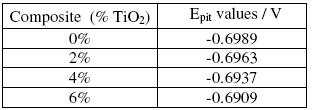
Table 3. Epit values of Al 6061 matrix alloy and Al 6061-TiO2 composites in 0.1 N NaCl solution.
Further physical examinations of the samples revealed the presence of less number of pits in unreinforced matrix as compared to composites, which can be attributed to increased area of reinforcements in composites [18].
Scanning electron micrograph images of Al 6061 matrix alloy before and after corrosion in 0.1 N NaCl medium are shown in Fig. 4 and Fig. 5, respectively. Scanning electron micrograph image of reinforcement TiO2 is shown in Fig. 6. The particle size of reinforcement TiO2 is about 100 nm, as observed in the SEM image. Following the polarization studies in chloride medium, samples were subjected to SEM analysis after usual pretreatment. The scanning electron micrograph images of the corroded samples of Al 6061-TiO2 composites are shown in Fig. 7.
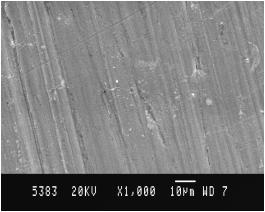
Figure 4. SEM of Al6061 matrix alloy.

Figure 5. SEM of corroded Al6061 matrix alloy in 0.1 N NaCl medium.

Figure 6. SEM of reinforcement TiO2.
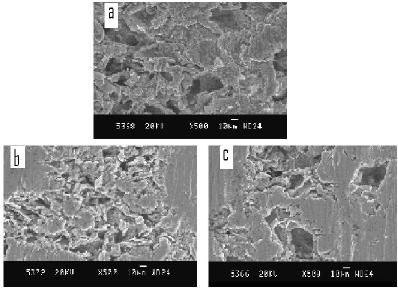
Figure 7. Scanning electron micrographs of corroded Al 6061-TiO2 composites samples in 0.1 N NaCl: a] SEM of Al 6061/ 6% TiO2 matrix alloy; b] SEM of Al 6061/ 4% TiO2 matrix alloy; c] SEM of Al 6061/ 2% TiO2 matrix alloy.
The scanning electron micrographs of corroded samples of matrix and composites reveal more severe pitting and cracks development in reinforced composites alloy than in unreinforced matrix. Greater degree of surface deterioration in composites as observed from SEM images indicates the higher corrosion rates for composites than for matrix alloy. The SEM micrographs show a complete deterioration of the smoothness of the surface of matrix [23], suggesting the penetration of chloride ions into the material surface forming corrosion spots [29].
EDX analysis of corroded samples of Al matrix alloy and composites after polarization studies in chloride medium are shown in Fig. 8. The peak due to Al, Mg and Si is less intense in the matrix alloy, while it increases with increase in reinforcement in the composites. The presence of large amounts of aluminum in the corrosion products formed on the samples of Al composites confirms the decreased corrosion resistance in Al composites with increase in the TiO2 content.
Figure 8.EDX examinations of: a) Al6061 matrix alloy; b) Al 6061/ 2% TiO2 composite; c) Al 6061/ 4%TiO2 composite; d) Al 6061/6% TiO2 composite.
Table 4 gives the electrochemical corrosion parameters for the samples in 0.1 N NaNO3 and 0.1 N Na2SO4 media. The polarization curves for Al matrix and its TiO2 composites in 0.1 N NaNO3 and 0.1 N Na2SO4 media exhibited the same trend as that of NaCl solution (hence not reproduced here).
Table 4. Electrochemical corrosion parameters of Al6061 and its TiO2 composites in 0.1 N NaNO3 and 0.1 N Na2SO4.
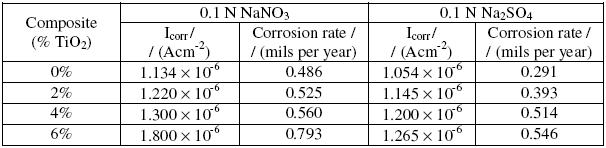
The high corrosion current densities and corrosion rates of Al6061 matrix and composites in chloride solution (Table 2), as compared to the values in nitrate and sulphate media, can be attributed to greater incorporation of chloride ions into the anion vacancies of the passive Al2O3 film [27] and a preferential penetration ability of chloride ion into the passive oxide layer. Chloride ions are more aggressive in pitting due to their adsorbability on the passive layer compared to sulphate and nitrate ions [20]. The adsorption of chloride leads to the breakdown of the protective oxide film [22] and TiO2/Al 6061 interface provides preferred sites for breakdown of the protective oxide layer. Low corrosion rates observed in both nitrate and sulphate media, for matrix as well as composites can be attributed to their ability to suppress both cathodic and anodic currents[21].
A comparison of the results from all the media indicates that the relative degree of corrosion in different ionic species follows the order Cl- > NO3- > SO42-. The lowest corrosion rate observed in SO42- medium is probably due to the formation of passive aluminium sulphates, which are less soluble than other salts formed in the presence of Cl- and NO3- ions.
Conclusions
The corrosion behaviour of unreinforced Al6061 matrix and TiO2 particulate-reinforced composites (2, 4 and 6% by weight) in various media like NaCl, NaNO3, Na2SO4 and HCl has been investigated using potentio-dynamic polarization technique and weight-loss methods. Results of our investigation point out to the following:
1. Corrosion current values (Icorr) increase with increase in TiO2 content in the composites up to 6% by weight. This indicates a nominal increase in the corrosion rate of the matrix alloy upon reinforcement with TiO2.
2. The decreased corrosion resistance in composites is believed to be due to the possible microgalvanic coupling between the conducting interfacial products and the matrix alloy.
3. A comparison of the corrosion parameters of the matrix alloy and its composites in different salts and acid media shows that the corrosion rate is highest in chloride and least in the sulphate medium.
4. The scanning electron micrographs of corroded samples of matrix and composites reveal more severe pitting and cracks development in reinforced composites than in unreinforced matrix alloy.
5. EDX analysis of corroded samples confirms the decreased corrosion resistance in Al composites with increase in the TiO2 content.
6. Low corrosion rates observed in both nitrate and sulphate media, for matrix as well as composites can be attributed to their ability to suppress both cathodic and anodic currents.
Acknowledgements
The authors are grateful to the Principal, the Director & the Managements of Dr.Ambedkar Institute of Technology and RNS Institute of Technology, Bangalore, for their support and encouragement towards our research work.
References
1. J.E. Allison, G.S. Cole, J. Met. 45 (1993) 19-24.
2. C.G.E. Mangin, J.A. Isaacs, J.P. Clark, J. Met. 48 (1996) 49-51.
3. H.G. Kang, D.L. Zhang and B. Cantor, J. Microstrc. 169 (1993) 239-245.
4. M.S.N. Bhat, M.K. Surappa, N. Sudhaker, J. Mat. Sci. 26 (1991) 4991-4996. [10.1007/BF00549882]
5. S.L. Pohlman, Corrosion 34 (1978) 156-159.
6. P.P. Trzaskoma, E. McCafferty, C.R. Crowe, J. Electrochem. Soc. 130 (1983) 1804–1809. [10.1149/1.2120102]
7. A. Albiter, A. Contreras, M, Salazar, J.G. Gonzalez-Rodriguez, J. Appl. Electrochem. 36 (2006) 303-308. [10.1007/s10800-005-9073-z]
8. J.B. Fogangolo, M.H. Robert, Ruiz-Navas, J.M. Torralba, J. Mat. Sci. 39 (2004) 127-132. [10.1023/B:JMSC.0000007736.03608.e5]
9. K.H.W. Seah, M. Krishna, V.T. Vijayalashmi, J. Uchil, Corros. Sci. 44 (2002) 917-925. [10.1016/S0010-938X(01)00099-3]
10. S.C. Sharma, Corros. Sci. 43 (2001) 1877-1889. [10.1016/S0010-938X(00)00186-4]
11. P.V. Krupakara, S. Manjunatha, M. Krishna and J. Uchil, presented at ADCOMP, 2000, 24-26, August 2000, Bangalore.
12. S.C. Sharma, K.H.W. Seah, B.M. Sathish, B.M. Girish, Corros. Sci. 39 (1997) 2143-2150. [10.1016/S0010-938X(97)00098-X]
13. J.M.G. De Salazar, S. Urena, S. Manzanedo, M.I. Barrena, Corros. Sci. 41 (1991) 529 -545. [10.1016/S0010-938X(98)00135-8]
14. A.A. Mazhar, W.A. Badawy, Abou-Romia, Surf. Coat. Technol. 29 (1986) 335. [10.1016/0257-8972(86)90006-X]
15. P.L. Cabot, F.A. Centellas, J.A. Garrido, E. Perez,H. Vidal, Electrochim. Acta 36 (1991) 179-187. [10.1016/0013-4686(91)85199-H] [ Links ]
16. W.M. Carroll, C.V. Breslin, Br. Corros. J. 26 (1991) 255.
17. T. Das, P.R. Munroe, S. Bandyopadhyay, J. Mater. Sci. 31 (1996) 5351. [10.1007/BF01159304]
18. V.B. Raju, H.C.A. Murthy, H.B. Lokesh, Mater. Sci. Indian J. 4 (2008) 328-334.
19. P.P. Trzaskoma, E. McCafferty, Aluminium surface treatment technology, The Electrochemical Society, Pennington,NJ, (1986), 171.
20. L. Tomcsanyi, K. Varga, I. Bartik, G. Horanyi, E. Maleczki, Electrochim. Acta 34 (1989) 855-859. [10.1016/0013-4686(89)87119-1]
21. J. Datta, B. Samantha, A. Jana, S. Sinha, C. Bhattacharya, S. Bandyopadhyay, Corros. Sci. 50 (2008) 2658-2668. [10.1016/j.corsci.2008.06.027]
22. Z. Ahmad, P.T. Paulette, B.J.A. Aleem, J. Mater. Sci. 35 (2000) 2573-2579. [10.1023/A:1004767113052]
23. J. Datta, C. Bhattacharya, Bandyopadhyay, Bull. Mater. Sci. 28 (2005) 253-258.
24. Z.S. Liu, B.T. Wu, M.Y. Gu, J. Mater. Sci. 42 (2007) 5736-5741. [10.1007/s10853-006-0754-8]
25. J. Bienias, M. Walczak, B. Surowska, J. Sobczak, J. Optoelectronics Adv. Mater. 5 (2003) 493-502.
26. I. Gurrappa, V.V. Bhanu Prasad, Mater. Sci. Technol. 22 (2006) 115-122.
27. Z. Szklarska-Smialowska, Corros. Sci. 41 (1999) 1743-1767. [10.1016/S0010-938X(99)00012-8]
28. L. Pohlman, Corrosion 34 (1978) 156-159.
29. W.A. Badawy, F.M, Al-Kharafi, A.S. El-Azab, Corros. Sci. 41 (1999) 709-727. [10.1016/S0010-938X(98)00145-0]
30. R.G. Buchheit, J. Electrochem. Soc. 142 (1995) 3994-3996. [10.1149/1.2048447]
Received 03 March 2010; accepted 11 November 2010
*Corresponding author
E-mail address: rajuvbait@gmail.com














