Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portugaliae Electrochimica Acta
versão impressa ISSN 0872-1904
Port. Electrochim. Acta v.28 n.4 Coimbra 2010
Removal of Heavy Metals (Fe3+, Cu2+, Zn2+, Pb2+, Cr3+ and Cd2+) from Aqueous Solutions by Using Hebba Clay and Activated Carbon
S.A. Shama,1, * M.A. Gad2
1 Chemistry Department, Faculty Of Science, Benha University, Egypt
2 Suez Regional Branch, Egyptian Environmental Affairs Agency (EEAA), Egypt
DOI: 10.4152/pea.201004231
Abstract
The adsorption capacity of hebba clay and activated carbon towards (Fe3+, Cu2+, Zn2+, Pb2+, Cr3+, Cd2+) metal ions was studied. The adsorption capacity was investigated by batch experiment. The effect of weight of hebba was studied and the results showed that the removal percentages increased as the weight of sorbent increased. The effect of contact time was also studied and the results showed that the removal percentages increased as the contact time increased. The effect of pH of the solution was also studied and the removal percentages for (Cu2+, Zn2+ and Cd2+) were affected slightly by changing the pH value, but for (Fe3+, Pb2+ and Cr3+) the effect was higher. Also, the effect of initial concentration of metal ions was studied at four different concentrations (5, 10, 30, 50 mg/L); in case of metal ions (Cu2+, Zn2+ and Cd2+), the removal percentages increased by increasing initial concentration. But for the other metal ions it decreased.
The order of increasing removal percentages of metal ions at pH=4.86, concentration of metal ions 30 mg/L, and after four hours of shaking, was (Pb2+ < Cu2+ < Cd2+ < Cr3+ < Zn2+ < Fe3+). But in the case of activated carbon, the order was Cd2+ < Zn2+ < Cu2+ < Pb2+ < Cr6+ < Fe3+.
Keywords: removal, heavy metal, hebba clay, activated carbon, aqueous solution, atomic absorption spectrophotometer.
Introduction
Activated sludge is used as bioadsorbent for Cu2+, Cd2+ and Ni2+. Pretreatment with NaOH was found to improve the adsorption capacity of the sludge, whereas its treatment with HCl reduces it. [1]. Anaerobic sludge supplied from wastewater treatment plants, acts as a novel biosorbent for Pb2+, Cu2+, Cd2+, and Ni2+ removal from aqueous solutions [2]. Rice husk, a surplus agricultural byproduct, is used for the sorption of Cd2+ from aqueous solution. Some simple and low-cost chemical modifications which resulted in increasing the sorption capacity of raw rice husk (RRH) have been studied [3]. The leached manganese nodules residue is considered as adsorbent for removal of heavy metal ions from contaminated water bodies [4]. A new class of nano-sized large pored titanium silicate variety, ETS-10 and ETAS-10 with different Al2O3/TiO2 ratio was successfully synthesized and applied to the removal of heavy metals, in particular, Pb2+, Cd2+, Cu2+, Co2+, Mn2+ and Zn2+ [5,6]. Unextracted residue obtained after a countercurrent two-step extractive process of silica from pumice lapillus, at 100 °C and room pressure, has been found mainly crystallized to the pseudo-cubic form typical of zeolite P. This residue could be active as a low-cost agent for the removal of heavy metals from wastewater; the adsorption mechanism mainly involves an ionic exchange between sodium ions from the solid phase and heavy metals in solution [7]. Papaya wood was evaluated as a new biosorbent of heavy metals such as Cu2+, Cd2+ and Zn2+ [8]. The sorption of lead, copper, cadmium, zinc and nickel by algae and characterization of biosorptive capacity were significantly affected by solution pH [9]. Coffee residues binding with clay as adsorbent (hereafter called CC-adsorbent) is utilized for removal of heavy metal ions in solution [10]. Cocoa shells (CS) have been identified as a very efficient natural sorbent to remove Pb2+ and other metal ions from acid soil leachates (ASL) [11]. Fungal biomass immobilized within a loofa sponge (FBILS) is used as a new biosorbent system to remove heavy metal ions such as Pb2+, Cu2+ and Zn2+ from aqueous solution [12]. Calcined phosphate (CP) is a good adsorbent for the removal of Pb2+, Cu2+, and Zn2+ from solutions. The abundance of natural phosphate, its low price and non-aggressive nature towards the environment, are advantages for its utilization in the point of view of wastewater and wastes clean up [13].
Efforts have been done to minimize the production of hazardous waste; accumulated waste became one of the most important environmental challenges that the world faces today. So, the aim of the present work is to using cheap and undesirable materials like cotton stem (which is an agriculture byproduct) to produce activated carbon and the mineral clay hebba to remove heavy metal ions from wastewater, instead of the classical techniques that are difficulty controlled and mostly requiring expensive equipments.
Experimental
Adsorbent material
The adsorbent material (hebba clay and activated carbon) was collected from its origin sites and stored in polyethylene bags and then transported to the laboratory in an icebox within the limited time. The clay was prepared by air drying and crushing by using mortar and pestle and it was sieved by passing through a 60-mesh sieve.
Chemicals
1-Buffer solutions of pH equal to 4.01, 6.86 and 9.18 for calibration of the pH-meter.
2-Concentrated nitric acid 63%.
3-Individual stock standard solution of nitrate salt of Fe3+.
4-Individual stock standard solution of nitrate salt of Cu2+.
5-Individual stock standard solution of nitrate salt of Zn2+.
6-Individual stock standard solution of nitrate salt of Pb2+.
7-Individual stock standard solution of nitrate salt of Cr3+.
8-Individual stock standard solution of nitrate salt of Cd2+.
9-Sodium hydroxide pellets.
All chemicals used were high-grade chemicals from (Merck, BDH and Fisher) companies.
Chemical analysis
Determination of heavy metals
Heavy metals under study were determined by atomic absorption techniques, under appropriate conditions using an Atomic Absorption Spectrophotometer type AA-6800, Shimadzo, Japan.
Batch adsorption experiments
Cotton stem, as a cheap material, was chosen as a precursor for preparation of activated carbon by one step chemical activation using H3PO4 [1]. In this concern, a known weight of the crushed cotton stems was soaked in 85% (w/w) H3PO4. Then the volume of H3PO4 must be enough to cover the stems completely and the system was slightly agitated to ensure complete penetration of the acid through out the stem. The mixture was heated to 80 °C for one hour and left over night at room temperature to help appropriate wetting and impregnation of the precursor. The impregnated mass was dried in an air oven at 80 °C over night then placed in an electric furnace and the temperature raised at a rate of 10 °C/min up to 500 °C then left for 2.5 hours. The product was washed thoroughly with hot distilled water till pH=5 and finally dried at 110 °C; then grinded and sieved using a 60 mesh sieve.
Effect of weight of hebba clay on the removal of the heavy metal ions
A multi-element standard solution containing (Fe3+, Cu2+, Zn2+, Pb2+, Cr3+ and Cd2+) ions, whose concentrations were 30 mg/L, was prepared. The pH of the standard solution was adjusted to 4.86. To a 50 mL of the multi-element standard, (0.1, 0.2, 0.3 and 0.4 gm) of the hebba clay were added in an Erlenmeyer flask and the mixtures were shaked using a rotary shaker at about 100 rpm for 4 hours. After that the mixtures were filtered using a 0.45 µm filter paper. The filtrate and the multi-element standard were analyzed using an atomic absorption spectrophotometer.
Effect of contact time on the removal of the heavy metal ions
A multi-element standard solution was previously prepared. To a 50 mL of each solution, 0.4 g of the hebba clay were added and the mixtures were shaked for 2, 4 and 8 hours, and analyzed using an atomic absorption spectrophotometer.
Effect of pH on the removal of the heavy metal ions
A series of standard solutions was prepared at different pH values (2.06, 3.77 and 4.86), treated as previously mentioned, and analyzed using an atomic absorption spectrophotometer.
Effect of initial concentration of the heavy metal ions on the removal percentage
A series of multi-element standard solutions whose concentrations were (5, 10, 30 and 50 mg/L) was prepared. The solutions were treated as previously and analyzed using an atomic absorption spectrophotometer.
Results and Discussion
Effect of weight of hebba clay on the removal of the heavy metal ions
The experiments were carried out with 0.1, 0.2, 0.3 and 0.4 g of the hebba clay added to prepared standard solutions (synthetic waste water); these four solutions were given the codes 1H, 2H, 3H and 4H for heba clay and 1C, 2C, 3C and 4C for activated carbon, respectively.
The effect of weight of sorbent (activated carbon) on the removal percentage of Fe3+, Pb2+, Zn2+, Cr3+, Cu2+ and Cd2+ is shown graphically in Fig. 1. Inspection of the data obtained showed that:
1-Maximum removal percentage was obtained for Fe3+ ion, which is nearly equal to 99.99%; the removal percentage is significantly increased as the weight of sorbent used increases.
2-Minimum removal was obtained for Pb2+ ion, but it is slightly increased by the increase in the weight of sorbent clay.
3-The removal of Cd2+ is very slightly increased by the increase in the weight of sorbent.
4-The variation of maximum removal percentage of metal ions with weight of clay used as sorbent lies in the order Fe3+ > Zn2+ > Cr3+ > Cd2+ > Cu2+ > Pb2+, but in case of activated carbon lies in the order Fe3+ > Pb2+ > Cr6+ > Zn2+ > Cu2+ > Cd2+.
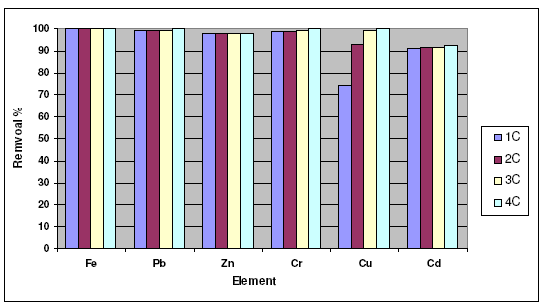
Figure 1. Effect of weight of activated carbon on the removal of heavy metal ions.
Effect of contact time on the removal of the heavy metal ions
The results are presented in Fig. 2, where it is found that the removal percentage for the six heavy metal ions (Fe3+, Pb2+, Zn2+, Cr3+, Cu2+ and Cd2+) increases as the contact time increases. The maximum removal value at the same contact time, e.g. 8 h, was for Fe3+, and the minimum value was for Pb2+. The decreasing order of the removal values at 8 h contact time is Fe3+ > Cr3+ > Zn2+ > Cd2+ > Cu2+ >P b2+, as the removal values were equal to 99.99 % > 95.63 % > 94.6 % > 89.95 % > 71.83 % > 63.47 %, respectively. On the other hand, the removal percentage in case of activated carbon for some metal ions like Fe3+, Pb2+and Cr6+ was not affected by changing the contact time.
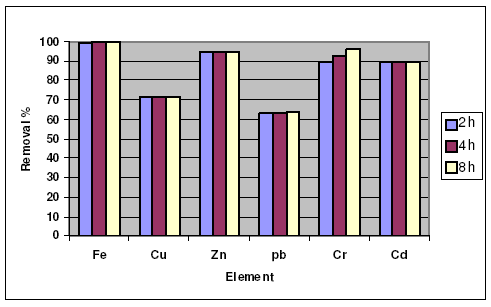
Figure 2. Effect of contact time of the heba on the removal of heavy metal ions.
Effect of pH on the removal of the heavy metal ions
For hebba clay as sorbent, the results are presented in Fig. 3, showing that the removal percentages increase as pH of the solution increases. In case of some metal ions the removal values increase greatly by increasing the pH value of the solution, such as Fe3+, Pb2+ and Cr3+, and in the other cases the removal values increase slightly as the pH of the solution increases, such as Cu2+, Zn2+ and Cd2+. The results also show the great adsorption affinity of hebba towards the heavy metal ions in slightly acidic medium. But in case of activated carbon, the results show that the maximum removal takes place at pH 4.86, which indicates that the maximum adsorption affinities take place in moderately and slightly acidic medium. Zn2+ ion has a notable affinity to be adsorbed on the activated carbon, in strongly, moderately and slightly acidic medium.
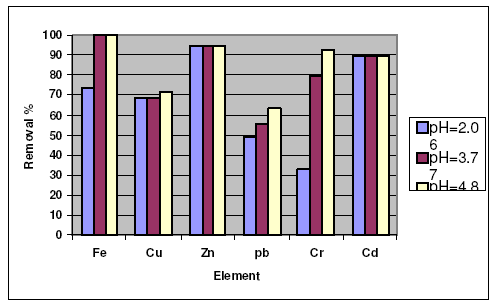
Figure 3. Effect of pH of the heba on the removal of heavy metal ions
Effect of initial concentration of the heavy metal ions on the removal percentage
The variation of removal percentage with change in initial concentration of heavy metal ions showed no regular trend. For example, Cu2+, Pb2+ and Cr3+ showed maximum adsorption at lower initial concentration, 5 mg/L, then decreased as the initial concentration increases up to 50 mg/L in case of heba clay, but in case of activated carbon, only Cr6+ ion showed no change in removal percentage.
On the other hand, the removal percentage of Zn2+ and Cd2+ increases as their initial concentrations increase in case of heba clay. For Fe3+ ion hebba attains maximum adsorption at initial concentration of 30 mg/L, then suffers a decrease in adsorption affinity at higher concentration. The results are presented in Fig. 4.
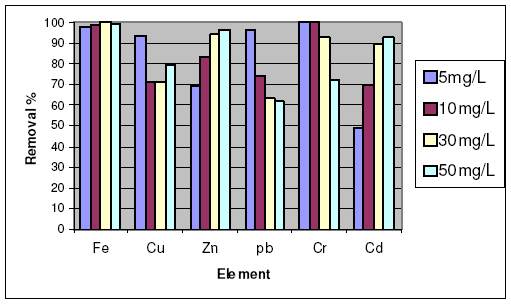
Figure 4. Effect of initial concentration of heavy metal ions on the removal percentage using heba.
Adsorption isotherms
Adsorption data for adsorbate concentration are most commonly described by adsorption isotherm, such as the Langmuir or Freundlish isotherms.
The Langmuir isotherm is valid for monolayer adsorption onto a surface containing a finite number of identical sites. The model assumes uniform energies of adsorption onto the surface and no transmigration of adsorbate in the plane of the surface. It is represented by the following equation:

where C is the concentration of solute remaining in solution at equilibrium (mg/L); qe is the amount of solute adsorbed per weight unit of solid adsorbent at equilibrium (mg/g); and Qo and b are Langmuir constants related to the adsorption capacity and energy of adsorption, respectively. These values can be obtained from the plot of 1/qe against (1/C).
The Langmuir linear relation is shown representatively in Fig. 5.
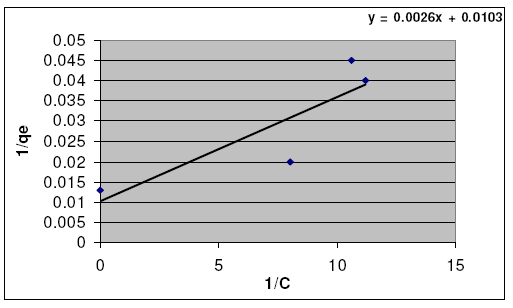
Figure 5. Langmuir curve for adsorption of Fe3+ ion on hebba clay.
The Freundlich adsorption isotherm was also applied for the adsorption of metal ions on hebba clay. The Freundlich equation is represented as:

or
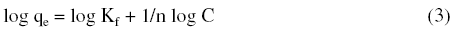
So, by plotting of Log qe vs. Log C, the constant Kf and exponent (n) can be determined.
Freundlich linear relation is shown in representative examples in Fig. 6.
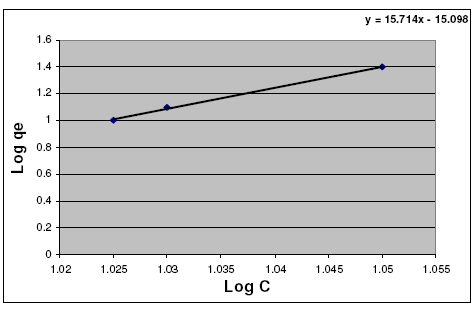
Figure 6. Freundlich curve for adsorption of Fe3+ ion on hebba clay.
Conclusion
It could be concluded that some low cost materials like hebba clay or activated carbon can be used efficiently in the removal of heavy metal ions (Pb2+, Cu2+, Cd2+, Cr3+, Zn2+ and Fe3+) from aqueous solutions. The removal of heavy metal ions was pH dependent, as the adsorption capacity increases with increasing pH value of the solution, and at a particular pH the order of increasing removal percentage was Pb2+ < Cu2+ < Cd2+ < Cr3+ < Zn2+ < Fe3+ using hebba clay. But, in case of activated carbon, the order of increasing removal percentage was Cd2+ < Zn2+ < Cu2+ < Pb2+ < Cr6+ < Fe3+.
The metal ions showed different behaviors towards adsorption on hebba clay by increasing the initial concentration of the metal ions. Adsorption of some metal ions were fitted with Langmuir isotherm, others with Freundlich isotherm, and other ones were fitted with both models. The experimental studies showed that hebba clay or activated carbon could be used as an alternative, inexpensive and effective material to remove high amounts of toxic heavy metal ions from wastewater.
References
1. Z. Al-Qodah, Desalination 196 (2006) 164-176. [10.1016/j.desal.2005.12.012] [ Links ]
2. A.H. Hawari, C.N. Mulligan, Biores. Tech. 97 (2006) 692-700. [10.1016/j.biortech.2005.03.033]
3. U. Kumar, M. Bandyopadhyay, Biores. Tech. 97 (2006) 104-109. [10.1016/j.biortech.2005.02.027]
4. N. Das, R.K. Jana, J. Colloid Interface Sci. 293 (2006) 253-262. [10.1016/j.jcis.2005.06.064]
5. J.H. Choi, S.D. Kim, S.H. Noh, S.J. Oh, W.J. Kim, Micropor. Mesopor. Mater. 87 (2006) 163-169. [10.1016/j.micromeso.2005.06.043]
6. J.H. Choi, S.D. Kim, Y.J. Kwon, W.J. Kim, Micropor. Mesopor. Mater. 96 (2006) 157-167. [10.1016/j.micromeso.2006.03.050]
7. P. Catalfamo, I. Arrigo, P. Primerano, F. Corigliano, J. Hazard Materials B134 (2006) 140-143. [10.1016/j.jhazmat.2005.10.040]
8. A. Saeed, M.W. Akhter, M. Iqbal, Sep. Purifi. Tech. 45 (2005) 25-31. [10.1016/j.seppur.2005.02.004]
9. P.X. Sheng, Y.P. Ting, J.P. Chen, L. Hong, J. Colloid Interface Sci. 275 (2004) 131-141. [10.1016/j.jcis.2004.01.036]
10. V. Boonamnuayvitaya, C. Chaiya, W. Tanthapanichakoon, S. Jarudilokkul, Sep. Purifi. Tech. 35 (2004) 11-22. [10.1016/S1383-5866(03)00110-2]
11. N. Meunier, J.F. Blais, R.D. Tyagi, Hydrometallurgy 73 (2004) 225-235. [10.1016/j.hydromet.2003.10.011]
12. M. Iqbal, R.G.J. Edyvean, Min. Eng. 17 (2004) 217-223. [10.1016/j.mineng.2003.08.014]
13. A. Aklil, M. Mouflih, S. Sebti, J. Hazard. Materials A112 (2004) 183-190. [10.1016/j.jhazmat.2004.05.018]
Received 5 July 2009; accepted 31 August 2010
* Corresponding author. E-mail address: sashama92@yahoo.com














