Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portugaliae Electrochimica Acta
versão impressa ISSN 0872-1904
Port. Electrochim. Acta v.28 n.3 Coimbra 2010
Study on the Inhibition of Mild Steel Corrosion by Benzoisoxazole and Benzopyrazole Derivatives in H2SO4 Medium
K. Parameswari,* S. Rekha, S. Chitra, E. Kayalvizhy
P.S.G.R. Krishnammal College for Women, Coimbatore, Tamilnadu, India.
DOI: 10.4152/pea.201003189
Abstract
Four heterocyclic compounds, namely 4- phenyl-5-acetyl/carbethoxy-3-methyl-6-hydroxyl-6-methyl-4,5,6,7-tetrahydro-2,1-benzoisoxazole and benzopyrazole (BIS1, BP1and BIS2, BP2), were synthesized and their influence on the inhibition of corrosion of mild steel in 1 M H2SO4 was investigated by means of weight loss, potentiodynamic polarization, electrochemical impedance (EIS) and scanning electron microscopy (SEM). The values of activation energy and free energy of adsorption of these compounds were also calculated. Adsorption obeys Langmuir adsorption isotherm. The IE of the compounds was found to vary with concentration and temperature. Synergistic effect was also investigated for the four compounds at 0.05 mM concentration by weight loss method in 1 M H2SO4 medium in presence of KI, KBr and KCl. Results obtained revealed that all the four compounds performed excellently as a corrosion inhibitor for mild steel in 1 M H2S04 and their efficiency attains more than 90% at 0.6 mM at 298 K. Polarisation studies showed them to be mixed type inhibitors.
Keywords: corrosion inhibition, weight loss, potentiodynamic polarization, EIS, synergism SEM.
Introduction
It is well documented that, the development of new corrosion inhibitors of non-toxic type, which do not contain heavy metals and organic phosphates, is very important [1]. Inorganic compounds such as chromate, dichromate, nitrite and nitrate are widely used as corrosion inhibitors in several media and for different metals and alloys [2,3]. On the other hand, the biotoxicity of these products, especially chromate, is well documented [4] as well as their non-environmental-friendly characteristics [5] which limit their application. Among alternative corrosion inhibitors, organic compounds containing N, S and O have been reported as inhibitors which reduce the rate of the dissolution of metals [6, 7].
These organic compounds adsorb on metallic surface and decrease the corrosion rate. The most efficient inhibitors are compounds containing ? bonds in their structures. The adsorption of these compounds is influenced by the electronic structure of inhibiting molecules, steric factor, aromaticity and electron density at the donor site, presence of functional group such as –CHO,-N=N,R-OH, etc., molecular area and molecular weight of the inhibitor molecule [8].
N-heterocyclic compounds act by adsorption on the metal surface, and the adsorption takes place through nitrogen atom, as well as with triple or conjugated double bonds or aromatic rings in their molecular structures. Up to now, many N-heterocyclic compounds, such as imidazoline, triazole, pyrimidine, pyrrole , pyridine, etc., derivatives [9-12], have been used for the corrosion inhibition of iron or steel in acidic media. Even though isoxazolidines are easily accessible and known for many decades, only recently, the heterocyclic compounds containing NO moiety embedded in the five-membered ring were introduced to the corrosion literature for the first time [13]. Though the existing data show that numerous N-heterocyclic organic compounds have good anticorrosive activity, some of them are highly toxic to both human beings and environment. The safety and environmental issues of corrosion inhibitors arisen in industries has always been a global concern. These toxic effects have led to the use of eco-friendly and harmless N-heterocyclic compounds as inhibitors.
This work is aimed to study the effect of new synthesized benzopypyrazole and benzoisoxazole for inhibition of the corrosion of mild steel in 1 M H2SO4 by using techniques such as weight loss, potentiodynamic polarization, electrochemical impedance (EIS) and scanning electron microscopy (SEM). Synergistic effect was also carried out for mild steel samples in H2SO4 media with 0.05 mM concentration of the inhibitors and halides.
Experimental details
Preparation of inhibitor
Benzoisoxazole and benzopyrazole derivatives were synthesized by the procedure described by Rajanarendra et al. [14] and were characterized by IR spectra.
Solutions
1 M H2SO4 was prepared by dilution of analytical grade H2SO4 with distilled water and standardised with a standard base. The concentration range of the inhibitor used was 0.05 mM - 0.6 mM in the acid solution, and 1 M sulphuric acid was used as blank for comparison of results.
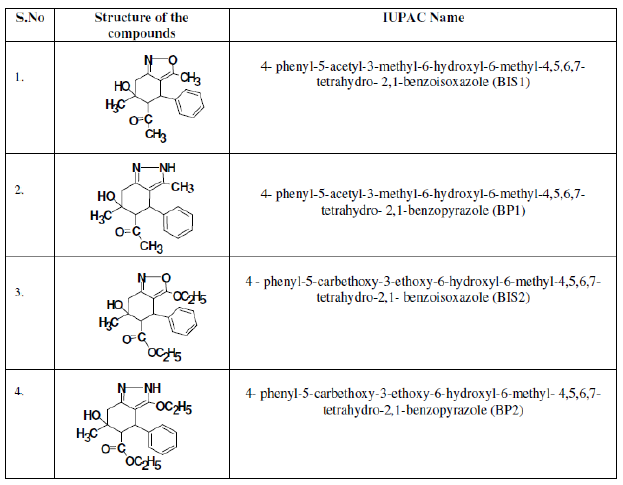
Table 1. Structure of the compounds.
Corrosion monitoring techniques
Weight loss method
Specimen
Weight loss experiments were performed with a cold rolled mild steel specimen of area 0.8113 inch2 having composition (Wt %) C-0.07%, S-Nil, P-0.008%, Si-Nil, Mn-0.34% and balance Fe; the specimen was polished using series of emery papers and finally degreased with organic solvent trichloroethylene and immediately used for experiments
Experimental procedure
Weight loss measurements were carried out by weighing the specimens before and after immersion in 100 mL acid solution for 3 hours in the absence and presence of inhibitors at various concentrations. Duplicate experiments (triplicate) were performed in each case and mean value of the weight loss was determined. From the initial and final masses of the specimen the weight loss was calculated. From this weight loss value, inhibition efficiency and corrosion rate were determined. Inhibition efficiency has been determined by the following relationship,
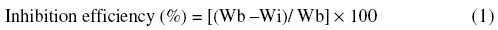
where Wb and Wi are the weight loss without and with inhibitor, respectively.
The corrosion rate has been determined by the relationship,
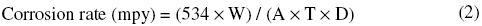
where W=weight loss in mg; A=area in inch2; T=time of immersion in sec; and D=density in g/cm3.
The surface coverage (Θ) has been determined using the following relationship:
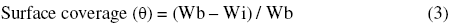
Temperature studies
The same procedure was carried out at different temperatures (303, 313, 323 and 333 K) using a thermostat to study the inhibition efficiency of the inhibitors at higher temperatures. This study gives details about the nature of adsorption and activation energy.
Synergistic effects of halide ions
The synergistic effect was studied in the presence of 1 mM KCl, KBr and KI to the steel specimen immersed for 3 hours in 1 M sulphuric acid containing 0.05 mM concentration of the inhibitors. The same weight loss method procedure has been followed to study the synergistic effect. From the weight loss data, the corrosion rate and the inhibition efficiency were calculated.
Electrochemical studies
Electrode surface preparation
The mild steel rod embedded in teflon with an exposed area of 0.8113 inch2 was polished using 1/0, 2/0, 3/0 and 4/0 grade emery papers and finally degreased with trichloroethylene and immediately used for the experiments.
Electrode cell assembly
Electrochemical measurements were carried out with a three electrode cell assembly. The working electrode was the mild steel rod. A saturated calomel electrode was used as the reference electrode. A rectangular Pt foil was used as the counter electrode. The solution capacity is 100 mL.
Procedure
Electrochemical impedance spectroscopy (EIS) and Tafel polarization were conducted in an electrochemical measurement unit (Model 1280 B Solartron, UK). The EIS measurements were made at a corrosion potential over a frequency range of 10 kHz to 0.01 Hz with signal amplitude of 10 mV. The Tafel polarization measurements were made after EIS for a potential range of -200 mV to +200 mV with respect to open circuit potential, at a scan rate of 1 mV/sec. The Icorr, Ecorr, Rct and Cdl values were obtained from the data using the corresponding “Corr view” and “Zview” software. The inhibition efficiency from potentiodynamic polarization was calculated from the value of Icorr by using the formula
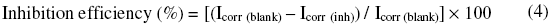
The inhibition efficiency from the impedance measurements was calculated using the formula
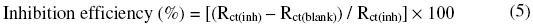
where Rct(inh) is the charge transfer resistance in the presence of inhibitor and Rct(blank) is the charge transfer resistance in the absence of inhibitor.
SEM studies
The surface morphology of the corroded and inhibited mild steel specimen was studied by SEM. The SEM photographs were recorded using a Geol scanning electron microscope. The mild steel specimen was suspended for 3hrs in blank H2SO4 and H2SO4 containing 0.6 mM of compound BIS1. The plates were washed with running water and dried used for SEM studies.
Results and discussion
Weight loss studies
The effect of addition of the benzopyrazoles and benzoisoxazoles tested at different concentrations on the corrosion of mild steel in 1 M H2SO4 was studied by weight loss method at 303 K after 3 hrs of immersion period. The values of corrosion rate and inhibition efficiency are given in the Table 2. For each compounds tested the I.E. increases with increasing concentration of the inhibitors (Fig. 1). The increase in efficiency with increasing the concentration is due to increase in surface coverage Θ, which shows that the compounds form a protective adsorptive layer on the surface. Thus adsorption forms the mechanism of inhibition.
Table 2. Inhibition efficiency of various concentrations of the inhibitors for the corrosion of mild steel in 1 M H2SO4 by weight loss method at 30 ± 1 °C.
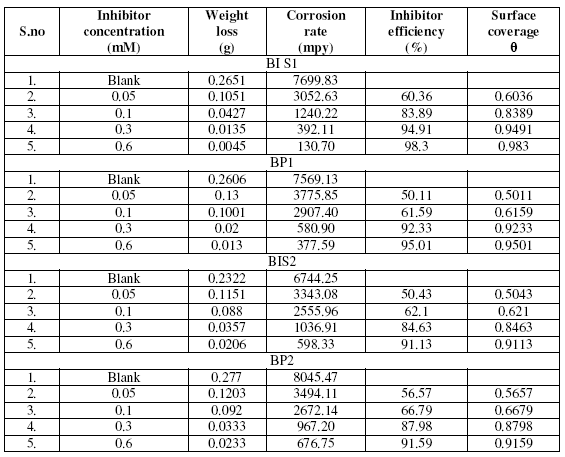
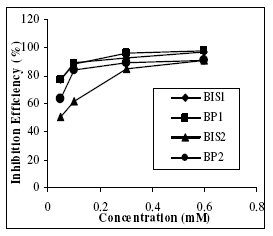
Figure 1. Variation of inhibition efficiency (%) vs. concentration of the inhibitors (mM).
Effect of temperature
To investigate the mechanism of inhibition and to determine the activation energies of the corrosion process, the weight loss studies were carried out at higher temperatures from 313-333 K. The results are given in Table 3. The inhibition efficiency decreases with temperature for all the inhibitors.
Table 3. Inhibition efficiency at 0.6 mM concentration of the inhibitors for the corrosion of mild steel in 1 M H2SO4 by weight loss method at higher temperatures.
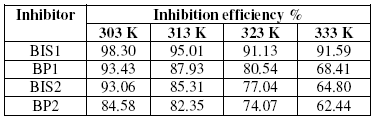
Fig. 1 presents the Arrhenius plots of log corrosion rate vs. 1/T for 1 M H2SO4 with and without the addition of the inhibitors and the slopes of the straight lines permit the calculation of Arrhenius activation energy, Ea.
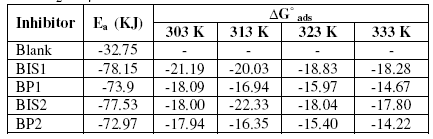
The values of Ea increase in the presence of inhibitors, which is shown in Table 4. Szauer, Brandt [15] and Foroulis [16] proposed that the lower activation energy value of the process in the presence of the inhibitor compared to that in its absence is attributed to chemisorptions, while the opposite is attributed to physical adsorption. The increase in Ea in the presence of benzopyrazoles and benzoisoxazoles indicates physical or weak bonding between the molecules of the inhibitor and the mild steel surface. The standard free energy of adsorption ΔG° ads can be calculated using the relation
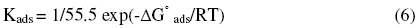
where 55.5 is the concentration of water in solution in moles / lit. The negative values of ΔG° ads (Table 4) ensure the spontaneity of the adsorption process and the stability of the adsorbed layer. Generally, values of ΔG° ads up to –20 KJ mol-1 are consistent with physical adsorption, i.e., electrostatic interactions between the charged molecules and charged metal surface, while those around -40 kJ mol-1 or higher are associated with chemisorptions as a result of sharing or transfer of electrons from organic molecules to metal surface [17].
Table 4. Activation energy Ea and free energy of adsorption (ΔG° ads) for the corrosion of mild steel in 1 M H2SO4 at 0.6 mM concentration of the inhibitors.
Adsorption isotherm
Adsorption isotherms are very important in determining the mechanism of organic electrochemical reactions. The most frequently used isotherms are Langmuir, Temkin and Frumkin. The compounds follow Langmuir adsorption isotherm, which is given as

Rearranging this equation

where Θ is the surface coverage degree, K the equilibrium constant of the adsorption process and C is the inhibitor concentration. It was found that a plot of C/ Θ vs. C is a straight line for all the inhibitors. Fig. 2 depicts the graph of the Langmuir adsorption isotherm for the studied compounds.
As adsorption is of Langmuir character, the organic molecules are attached as a monolayer and through a physical mechanism.
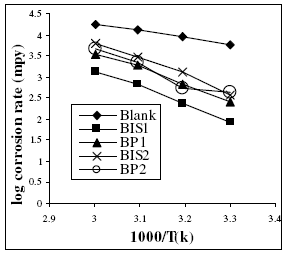
Figure 2. Arrhenius plot of corrosion rate of mild steel in 1 M H2SO4 solution in the absence and presence of 0.6 mM concentration of the inhibitors.
Polarization studies
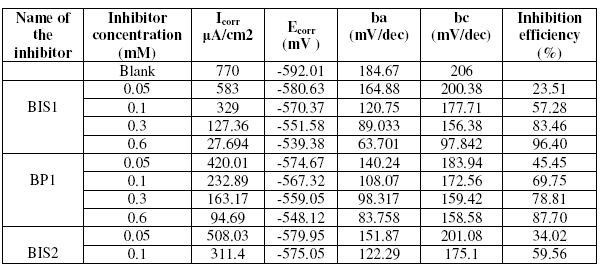
The inhibition process of the benzopyrazoles and benzoisoxazoles for the corrosion of mild steel in 1 M H2SO4 was analyzed by polarization experiments. Fig. 3 shows the Tafel anodic and cathodic polarization plots for the inhibitor BIS1. Table 5 gives the values of electrochemical corrosion parameters. It is evident that addition of inhibitors decreased the corrosion current density. The Ecorr value is shifted in the noble direction. Further, the Tafel slopes ba and bc are both decreased. But the anodic Tafel slopes are more decreased, showing that the inhibitors, being predominantly anodic, behave as mixed type.
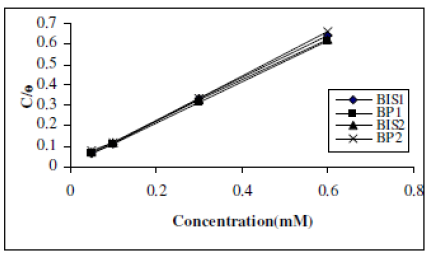
Figure 3. Langmuir plot of the inhibitors.
Table 5. Corrosion parameters for mild steel for various concentrations of the inhibitors in 1 M H2SO4 by potentiodynamic polarization studies.
EIS studies
The anticorrosive performance of the inhibitors was also studied by electrochemical impedance spectra at 30±1°C for various concentrations of the inhibitors in 1 M H2SO4. The data obtained are given in Table 6. The Rct is increased in the presence of the inhibitors. Maximum increase was observed for BIS 1 at 0.6 mM concentration.
Table 6. AC impedance parameters for mild steel for various concentrations of the inhibitors in 1 M H2SO4.
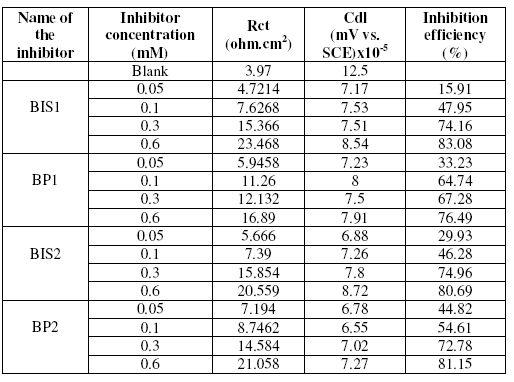
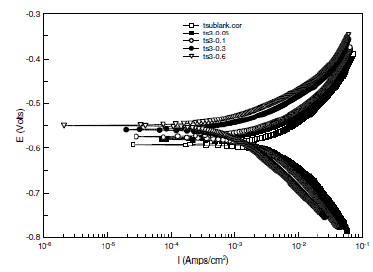
Figure 4. Potentiodynamic polarization curves for mild steel in 1 M H2SO4 in the absence and presence of selected concentrations of BIS1.
The double layer capacitance Cdl decreased, as concentration is increased. This decrease may be due to the adsorption of the compounds on the metal surface leading to a film formation. The corresponding Nyquist diagram for BIS1 is shown in Fig. 5. As can be noticed, the impedance diagrams are perfect semi-circles, indicating a charge transfer process mainly controlling the corrosion of the steel.
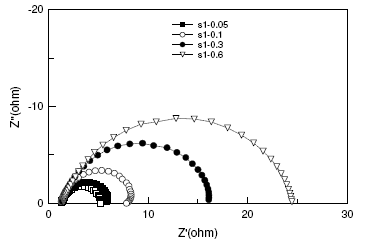
Figure 5. Nyquist plots for mild steel in 1 M H2SO4 in the absence and presence of selected concentrations of BIS1.
Synergistic effect of halide ion
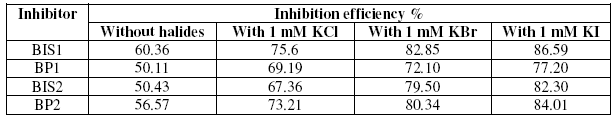
Synergism between the organic inhibitor and halide ions on metal corrosion in acidic solution has been researched by many authors [18]. Many studies indicate that nitrogen containing organic compounds have been found to behave better for the steel corrosion in hydrochloric acid than in sulphuric acid. The possible reason is that there is a synergistic inhibition between chloride ion and the nitrogen containing organic compounds [19]. In the present study, an attempt has been made to study the influence of halide ions on the corrosion inhibition of benzopyrazoles and benzoisoxazoles for mild steel in 1 M H2SO4, by weight loss measurements. The I.E. values obtained are given in Table 7. It is clear from the table that addition of halide ions enhanced the I.E. of all the four inhibitors.
Table 7. Synergistic effect of 1 mM KCl/1 mM KBr /1 mM KI on the inhibition efficiency of the inhibitors at 0.05 mM concentration by weight loss method.
Explanation for synergism
All the four compounds are nitrogen containing compounds, which contain unshared electron pair and Π-electrons. In strongly acidic solution, they may be protonated leading to a positively charged molecule. It is well known that steel surface contains positive charge due to Ecorr – Eq=0 > 0 [where Eq=0=potential of zero charge] [20]. Thus, it is difficult for the positively charged inhibitor molecules to approach the positively charged steel surface due to electrostatic repulsion. Addition of halide ions causes their specific adsorption on the steel and causes the steel surface negatively charged. The protonated organic inhibitors are then adsorbed by coulombic attraction on the metal surface and therefore their inhibition efficiency is increased. The synergistic effect increases in the order Cl- <Br -<I-. This is because iodide ion is the most adsorbable of halide ions on steel.
Evaluation of the inhibitors
Analysis of the inhibition efficiency of the compounds tested by all the methods shows the following order of efficiency: BIS1 > BIS2 and BP1 > BP2.
The two sets of compounds have identical general structure, except for the substituents at position 3 and 5 (Table 1). The higher I.E. of BIS1compared to BIS2, and BP1 compared to BP2, may be attributed to the presence of electron donating methyl group in BIS1 and BP1, which enhances the electron density on the isoxazole ring and pyrazole ring, respectively, which are the active sites of adsorption.
BIS2 and BP2 show somewhat lesser I.E. (by about 4-7%). This may be attributed to the steric interaction of the two bulky -OEt groups at position 3 and 5 with the phenyl ring at position 4. This steric repulsion makes the -OEt and COOEt groups to be out of the mild steel surface. Therefore, they have no influence on the electron density of the adsorption site (heterocyclic rings).
Comparison of I.E. of isoxazoles and pyrazoles (BIS1 and BP1, BIS2 and BP2) shows that the isoxazoles present slightly higher I.E. than the pyrazoles. Similar results were also reported by Swearingen and Schram [21] for the corrosion of mild steel in the presence of substituted amines. n-propylamine, ethylenediamine and ethanol amines were compared by their I.E. It was reported that the electronegative character of oxygen in ethanolamine results in a shift of electrons throughout the molecule in the direction of oxygen, making nitrogen center somewhat more positive and, as a result, more effectively adsorbed. Consequently, ethanolamine shows the same percentage of inhibition efficiency as compared to ethylamine. In the present set of compounds, also the electron withdrawing power of the oxygen reduces the e- donating power of the N by a large amount. However, the presence of the three lone pair of electrons in the adjacent atoms N-O in isoxazole ring makes this moiety more polarizable and a powerful nucleophile. The protonated conjugate acid >N+H-O- being weak may be involved in dynamic equilibrium with the neutral isoxazoles. While the neutral species get adsorbed on the anodic sites through interaction of lone pair of electron on N,O and aromatic p-electrons with Fe2+, the cationic species adsorb on the cathodic sites to decrease the evolution of hydrogen. Hence, more adsorption and inhibition. Similar explanation was given by Ali et al. [22] for the higher inhibition efficiency of bisisoxazolidines.
SEM
SEM photographs obtained for mild steel surface immersed in 1 M H2SO4 solutions for 3 hrs in the absence and presence of 0.6 mM of BIS1 are shown in Fig. 6 (a, b). It can be observed from Fig 6b, that the specimen surface was strongly damaged in the absence of the inhibitor. SEM image of inhibited mild steel specimen (Fig. 6a) reveals that a good protective adsorbed film is formed on the specimens surface, which suppresses the rate of corrosion, being responsible for the inhibition.
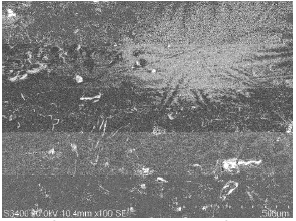
Figure 6a. SEM photograph of mild steel specimen after being corroded in 1 M H2SO4 solution for about 3hrs containing 0.6 mM of BIS.

Figure 6b. SEM photograph of mild steel specimen after being corroded in 1 M H2SO4 solution for about 3hrs.
Conclusions
- Benzoisoxazole and benzopyrazole derivatives exhibit excellent inhibition efficiency towards corrosion of mild steel in1 M H2SO4.
- The optimum inhibition efficiency of these compounds was achieved at the concentration 0.6 mM.
- The inhibitors were adsorbed on the mild steel by physisorption mechanism.
- The adsorption of inhibitors on mild steel in 1 M H2SO4 obeys Langumuir adsorption isotherm.
- Potentiodynamic polarization studies suggest that the inhibitors are mixed type ones.
- The SEM proves the formation of a protective film of the inhibitors.
- The order of synergism observed due to the added halides is KCl<KBr<KI.
References
1. E.S. Ferreira, C. Giacomelli, F.C. Giacomelli, A. Spinelli, Mater. Chem. Phys. 83 (2004) 129-134. [10.1016/j.matchemphys.2003.09.020]
2. M.G. Fontana, Corrosion Engineering, third ed., McGraw-Hill, Singapore, 1986.
3. Y. Abboud, A. Abourriche, T. Saffaj, M. Berrada, M. Charrouf, A. Bennamara, H. Hannache, Desalination 237 (2009) 175-189. [10.1016/j.desal.2007.12.031]
4. J. Sinko, Prog. Org. Coat. 42 (2001) 267-282. [10.1016/S0300-9440(01)00202-8]
5. S.E. Manahan, Environmental Chemistry, sixth ed., Lewis, Boca Raton, 1994.
6. O. Krim, M. Bouachrine, B. Hammouti, A. Elidrissi, M. Hamidi, Port. Electrochim. Acta 26 (2008) 283-289. [10.4152/pea.200803283] [ Links ]
7. M.A. Quraishi, S.K. Shukla, Mater. Chem. Phys. 113 (2009) 685-689. [10.1016/j.matchemphys.2008.08.028]
8. Y. Yan, W. Li, L. Cai, B. Hou, Electrochim. Acta 53 (2008) 5953-5960. [10.1016/j.electacta.2008.03.065]
9. F. Bentiss, M. Traisnel, L.Gengembre, M. Lagrenee, Appl. Surf. Sci. 161 (2000) 194-202. [10.1016/S0169-4332(00)00287-7]
10. J. Cruz, R. Martez, J. Genesca, E. Garcia-Ochoa, J. Electroanal. Chem. 566 (2004) 111-121. [10.1016/j.jelechem.2003.11.018]
11. M. Bouklah, A. Ouassini, B. Hammouti, A. El Idrissi, Appl. Surf. Sci. 250 (2005) 50-56. [10.1016/j.apsusc.2004.12.021]
12. A. Popova, M. Christov, S. Raicheva, E. Sokolova, Corros. Sci. 46 (2004) 1333-1350. [10.1016/j.corsci.2003.09.025]
13. S.A. Ali, M.T. Saeed, S.U. Rahman, Corros. Sci. 45 (2003) 253-266. [10.1016/S0010-938X(02)00099-9]
14. E. Rajanarendar, E.K. Rao, D. Karunakar, Ind. J. Chem. 43(B) (2006) 805-807.
15. T. Szauer, A. Brandt, Electrochim. Acta 26 (1981) 1253-1256. [10.1016/0013-4686(81)85107-9]
16. Z.A. Foroulis, Proceedings of the seventh European symposium corrosion inhibitors, Ferrara,1990,149.
17. F.M. Donahue,K. Nobe, J. Electrochem. Soc. 112 (1965) 886-891. [10.1149/1.2423723]
18. E. Khamis, E.S.H. El-Ashry, A.K. Ibrahim, Br. Corros. J. 35 (2000) 150-154.
19. Xueming Li, Libin Tang, Lin Li, Guannan Mu, Guangheng Liu, Corros. Sci. 48 (2006) 308-321. [10.1016/j.corsci.2004.11.029]
20. K.M. Ye, Mater. Protect. (Chinese) 23 (1990) 37-42.
21. Lloyyd E.Swearingen, Alfred F. Schram,(Pg. 180) (Ph.D. thesis, Graduate College of Lahom University)
22. S.A. Ali, H.A. Al-Muallem, S.U. Rahman, M.T. Saeed, Corros. Sci. 50 (2008) 3070-3077. [10.1016/j.corsci.2008.08.011]
Received 25 January 2010; accepted 24 June 2010
* Corresponding author: parampps@yahoo.co.in














