Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Nascer e Crescer
versión impresa ISSN 0872-0754versión On-line ISSN 2183-9417
Nascer e Crescer vol.28 no.3 Porto set. 2019
https://doi.org/10.25753/BirthGrowthMJ.v28.i3.16677
ORIGINAL ARTICLES | ARTIGOS ORIGINAIS
Serum sickness-like reaction in pediatric age - experience of an imunoalergology unit
Doença do soro-like em idade pediátrica - experiência de uma unidade de imunoalergologia
Francisca MartinsI, Tatiana PereiraII, Diana SoaresIII, Jorge RomarizIII, Fátima PraçaIII, Herculano CostaIII, Cláudia PedrosaIII
I - Department of Pediatrics, Unidade Local de Saúde do Alto Minho. 4904-858 Viana do Castelo, Portugal. franciscamartins8@hotmail.com
II - Department of Pediatrics, Centro Hospitalar Entre o Douro e Vouga. 4520-161 Santa Maria da Feira, Portugal. taty.pereira.0@gmail.com
III - Department of Pediatrics, Allergy Unit, Centro Hospitalar Vila Nova de Gaia/Espinho. 4434-502 Vila Nova de Gaia, Portugal. dianarsoares@gmail.com; jaromariz@gmail.com; fatimapraca@sapo.pt; herculano.costa@chvnge.min-saude.pt; claudiampedrosa@yahoo.com
Endereço para correspondência | Dirección para correspondencia | Correspondence
ABSTRACT
Background: Serum sickness is a systemic reaction reported after administration of heterologous serum. It is clinically characterized by fever, skin lesions, arthralgia/arthritis, lymphadenopathy, and nephritis and by presence of immune complexes. Serum sickness-like reaction mimics serum sickness and its pathophysiology is not well understood. It is more common in pediatric age and often associated with drug administration. This study aimed to characterize serum sickness-like reaction cases evaluated at a Drug Allergy Clinic.
Material and methods: An analytical, retrospective, longitudinal study of a sample of 39 children (56% male) with diagnosis of serum sickness-like reaction evaluated at a Drug Allergy Clinic between January 2007 and December 2017 was performed.
Results: Children had an average of 4.8 years at the time of reaction. In most cases (97%), beta-lactam antibiotics were the suspected triggering drugs. On average, clinical manifestations developed 7.5 days after treatment start. Skin lesions developed in all patients, and arthralgia/arthritis in 92.3%. Fifteen percent of children were hospitalized. Penicillin-specific IgE was evaluated in 87% of patients, but only tested positive in two. Skin prick tests and intradermal tests were performed in 46% of cases and were positive in seven. Oral challenge test was performed in 26% of patients and was positive in three.
Conclusion: Serum sickness-like reaction is a rare and poorly understood entity. Diagnosis is essentially clinical, requiring a high index of suspicion. In the acute phase, usefulness of complementary tests lies mainly in the differential diagnosis with other diseases.
Keywords: antibiotics; children; serum sickness-like reaction
RESUMO
Introdução: A doença do soro é uma reação sistémica descrita após administração de soro heterólogo, caracterizada clinicamente por febre, lesões cutâneas, artralgias/artrite, linfadenopatia e nefrite, e laboratorialmente por presença de imunocomplexos. A doença do soro-like mimetiza a doença do soro, não estando a sua fisiopatologia bem estabelecida. É mais frequente em idade pediátrica e surge muitas vezes associada à administração de fármacos. Este estudo pretendeu caracterizar os casos de doença do soro-like avaliados numa consulta de alergia a fármacos.
Material e métodos: Este foi um estudo analítico, retrospetivo e longitudinal de uma amostra de 39 crianças (56% do sexo masculino) com diagnóstico de doença do soro-like avaliadas em consulta de alergia a fármacos entre janeiro de 2007 e dezembro 2017.
Resultados: A idade média das crianças na altura da reação foi de 4,8 anos. Antibióticos beta-lactâmicos foram os fármacos suspeitos na maioria dos casos (97%). As manifestações clínicas surgiram, em média, 7,5 dias após o início do tratamento. Verificaram-se lesões cutâneas em todos os doentes e atingimento articular em 92,3%. Um total de 15% das crianças foram internadas. Foi efetuado doseamento de IgE específicas em 87% dos doentes, tendo sido positivo em apenas dois. Foram realizados testes cutâneos por picada e intradérmicos em 46% dos casos, com resultados positivos em sete. A prova de provocação oral foi realizada em 26% dos doentes, tendo sido positiva em três.
Conclusão: A doença do soro-like é uma patologia rara e pouco estudada. O diagnóstico é essencialmente clínico, sendo necessário um elevado índice de suspeição. A utilidade dos exames complementares reside principalmente no diagnóstico diferencial com outras patologias.
Palavras-chave: antibióticos; crianças; doença do soro-like
Introduction
First described in humans in 1905 by Pirquet and Schick, serum sickness (SS) is a systemic reaction that occurs after administration of heterologous serum containing proteins of non-human species, usually of animal origin.1 It is a type III hypersensitivity reaction mediated by immune complexes (IC) deposited on the vascular walls, activating the complement cascade with consequent release of inflammatory mediators and tissue damage.2-4 The clinical picture is characterized by exanthema, arthralgia/arthritis, fever, lymphadenopathy, nephritis with albuminuria and hematuria and, more rarely, hepatosplenomegaly. Laboratory findings include circulating immune complexes and hypocomplementemia.2,3
Serum sickness-like reaction (SSLR) mimics SS symptoms, being more common among the pediatric population. It is often associated with drug administration, usually antibiotics, but other etiologies, such as infections and vaccines, may be involved. The underlying pathophysiological mechanisms remain unclear, but ICs are not detectable in most cases. Consequently, SSLR does not usually present with hypocomplementemia, vasculitis, or nephritis. Usually, it occurs one to three weeks after exposure to a given agent and typical clinical manifestations include fever, mostly urticarial rash, and polyarthralgia.3,5-7
Diagnosis is essentially clinical, being determined by the temporal relationship between drug ingestion and symptomatology onset. Auxiliary diagnostic exams are usually not required, as they are not associated with characteristic laboratory abnormalities.8 Hemogram is usually normal or evidencing neutropenia, leukopenia, lymphocytosis with plasmacytoid lymphocytes, eosinophilia, and mild thrombocytopenia. C-reactive protein and sedimentation rate may be slightly high. Hepatic cytolysis enzymes and renal function indexes are usually within the reference values. Since IC formation does not occur, these are usually not detected and the amount of complement fractions is normal. Urinalysis reveals no changes.3 Differential diagnosis includes viral infections (such as infectious mononucleosis, erythema infectiosum, or exanthema subitum), scarlet fever, erythema multiform, Kawasaky’s disease, Henoch- Schönlein purpura, juvenile idiopathic arthritis, acute annular urticaria, disseminated meningococcal disease, and reactive arthritis.3,9
Treatment consists of discontinuing the triggering agent and, if necessary, using symptomatic therapy with analgesics, non-steroidal anti-inflammatory drugs, antihistamines, and corticosteroids. Progression is usually benign and self-limiting, with complete resolution within seven to 21 days.3,10-12
The aim of this study was to characterize SSLR cases evaluated in a Pediatric Allergy Clinic.
Materials and methods
This was an analytical, retrospective, longitudinal study of 39 children evaluated at a Drug Allergy Clinic between January 2007 and December 2017, based on a data retrieved from children’s clinical records.
Analysed variables included age, gender, family and personal history of atopy, suspected drug and prescription reason, clinical manifestations, reaction timing, treatment, and allergy study results.
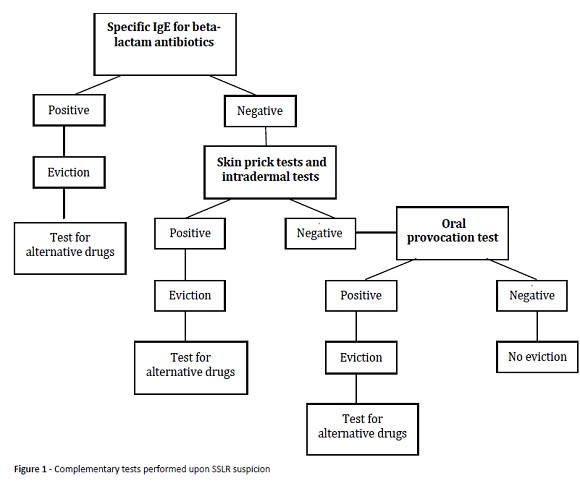
Since no guidelines are available in the literature concerning the most appropriate approach for patients with SSLR suspicion, the authors decided to perform a study based on complementary tests performed upon suspicion of hypersensitivity reactions to beta-lactam antibiotics (Figura 1).
Serum levels of specific IgE for beta-lactams (penicillin G, penicillin V, amoxicillin, ampicillin, and cefaclor) were determined by immunoenzymatic method, with results expressed in kU/L. The cut-off of 0.35 kU/L was considered positive. Skin prick tests (SPT) and intradermal tests were performed according to European Academy of Allergology and Clinical Immunology (EAACI) standards, and injectable formulations of drugs to be tested (Diater® formulations of PPL, MDM, amoxicillin, and clavulanic acid) were used up to maximum non-irritating dilutions, with saline as negative control and histamine hydrochloride at 10 mg/mL as positive control.
In absence of a previous severe systemic reaction and after obtaining informed consent from parents or caregivers, an oral provocation test (OPT) was performed at a day-care hospital, consisting on administration of increasing doses of the suspicious drug under medical supervision. Patients kept taking the medicine for the tested drug at home for up to eight days at the usual dose and frequency.
Data retrieved was organized and coded using SPSS 23.0 for Windows, and a descriptive analysis of variables under study was subsequently conducted.
Results
A sample of 39 patients was identified in a total of 7729 first visits, 56% of which male (n=22). Average age at reaction time was 4.8 years (median 4.2 years), with a minimum of seven months and a maximum of 14.8 years. Only 23.1% of patients (n=9) had a personal history of atopy and none had a previous personal or family history of drug allergy.
Beta-lactam antibiotics were the suspected drugs in most cases (97%, n=38): amoxicillin in 56.4% (n=22), amoxicillin with clavulanic acid in 35.9% (n=14), and cefaclor in 5.1% (n=2). One patient had a suspected allergy to clarithromycin. Drugs involved were mostly used in upper respiratory tract infections: 48.7% (n=19) in tonsillitis and 30.8% (n=12) in acute otitis media.
Clinical manifestations emerged on average 7.5 days after treatment start (median of eight days; range one to 18 days). Skin lesions were present in all children, arthralgias/arthritis in 92.3% (n=36), with gait refusal in 17.9% (n=7) and fever in 38.5% (n=15). The most frequent cutaneous manifestation was urticaria (56.4%, n=22), followed by maculopapular exanthema (33.3%, n=13) and urticaria multiforme (7.7%, n=3). Only one child reported vasculitis-suggestive changes. Rash was associated with pruritus in 69.2% of cases (n=27). Angioedema (lip and periorbital) was observed in two children.
Only 23.1% (n=9) of cases had a blood analysis during acute phase. Main alterations found included leucocytosis with neutrophilia and increased C-reactive protein. No changes were observed in the remaining tests, including in the complement study (C3 and C4 fractions).
Most children (89.7%, n=35) attended the Emergency Department and were treated with antihistamines (56.4%, n=22), corticosteroids (41%, n=16), and non-steroidal anti-inflammatory drugs (33.3%, n=13). Fifteen percent of children were hospitalized.
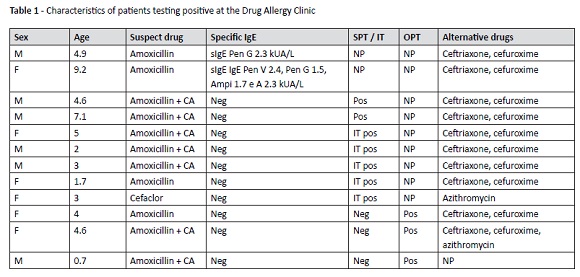
Regarding study performed at the Drug Allergy Clinic, determination of specific IgE for beta-lactam antibiotics was requested in 87% (n=34) of patients, resulting positive in two ( amoxicillin was the suspected drug). Penicillin G was positive for one patient and penicillin V, penicillin G, amoxicillin, and ampicillin were positive for the other patient.
SPT and intradermal tests were performed in 46% (n=18) of cases, with positive results in seven cases. For five patients, implicated drugs were amoxicillin and clavulanic acid in association. For the remaining two patients, suspected drugs were amoxicillin and cefaclor. All patients with positive skin tests were advised to maintain penicillin avoidance.
OPT was performed in 26% (n=10) of patients, and positive in three. In all cases, drugs tested were the suspected. In OPT positivity cases, drugs involved were amoxicillin and amoxicillin with clavulanic acid. In these patients, OPT was performed with an alternative drug (ceftriaxone, cefuroxime or azithromycin) and results were negative.
Nineteen patients refused to remain on the study and were discharged from the clinic with indication to avoid beta-lactam antibiotics, as hypersensitivity to these drugs was not possible to confirm or exclude.
Table 1 depicts a summary of the characteristics of patients with positive study results.
Discussion
In the present study, children’s median age was 4.2 years, which agrees with the literature indicating a high prevalence of SSLR below the age of five.11,13
Antibiotics, particularly beta-lactams, are the most frequently involved drug class, with cefaclor classically described as the most commonly implicated drug.3,5-7,11 In the present study, cefaclor was the suspected drug only in two patients, what may be explained by its lower use in this age group and geographical area.
Reactions developed on average 7.5 days after treatment, which is consistent with other studies reporting that the typical interval between drug administration and symptom onset is seven to 21 days.3,5-7,13,14
Also in agreement with the literature, in this study the most frequent clinical manifestations were rash − mostly urticarial and pruritus −, polyarthralgia, and fever. 3, 5-7, 13, 14 Absence of laboratory disease-specific changes described in other studies was also apparent in this study.8,13,14 Thereby, the usefulness of complementary diagnostic tests lies mainly in the differential diagnosis with other pathologies.
As noted, treatment consists of discontinuing the triggering agent and in symptom relief. A short corticosteroid cycle may be employed, although no formal recommendations are in place for its systematic use.3,10-12 Disease prognosis is generally favorable, although in more severe cases (fever >38.5ºC, disabling articular impairment, and extensive and petechial rash) hospitalization may be required.3 This occurred in 15% of children in the present study.
There is no consensus in the literature about the most appropriate approach to this type of reaction. SSLR does not meet indications for skin (prick or intradermal) or challenge tests, but they are also not contraindicated.15-19 One available option is determination of specific IgE and skin tests for beta-lactams, with OPT being the gold standard to establish or exclude an hypersensitivity diagnosis.18 Nevertheless, OPT with a suspected drug will only be performed if specific IgE and skin tests are negative, as in the present study. Performing OPT with alternative drugs allowed the identification of safe antibiotic alternatives for patients with confirmed SSLR.
In this group of patients, refusal to conclude the study remained significant, what may be partially explained by the age group in question and by the severity of clinical presentation.
Conclusion
SSLR is a rare and poorly studied entity. The most frequently implicated drugs are beta-lactam antibiotics. Diagnosis is essentially clinical, requiring a high index of suspicion, and determined by the temporal relationship between drug ingestion and symptom onset. In acute phase, usefulness of complementary tests lies mainly in the differential diagnosis with other pathologies. Such clinical cases should be referred to a specialized Drug Allergy Clinic, where a systematic approach is key for a correct diagnosis.
REFERENCES
1. von Pirquet CE. Allergy. Arch Intern Med 1911; 7: 259-88; 383- 436. [ Links ]
2. Lawley TJ, Bielory L, Gascon P, Yancey KB, Young NS, Frank MM. A prospective clinical and immunologic analysis of patients with serum sickness. N Eng J Med 1984; 311: 1407-13. [ Links ]
3. Mark HW. Serum sickness and serum sickness-like reactions. July, 2018. (Accessed August 8, 2018). Available in: http://www.uptodate.com . [ Links ]
4. Barata LT. Alergias. In: Arosa FA, Cardoso EM, Pacheco FC, editors. Fundamentos de imunologia. 1º edição. Lisboa: Lidel; 2007. p. 257-78. [ Links ]
5. Shin H, Chang M. Drug eruptions in children. Curr Probl Pediatr 2001; 205-30. [ Links ]
6. Segal AR, Doherty KM, Leggott J, Zlotoff B. Cutaneous reactions to drugs in children. Pediatrics 2007; 120: 1082-96. [ Links ]
7. Yorulmaz A, Akin F, Sert A, Agir MA, Yilmaz R, Arslan S. Demographic and clinical characteristics of patients with serum sickness-like reaction. Clin Rheumatol 2018; 37: 1389-94. [ Links ]
8. Tatum AJ, Ditto AM, Patterson R. Severe serum sickness-like reaction to oral penicillin drugs: three case reports. Ann Allergy Asthma Immunol 2001; 86: 330-4. [ Links ]
9. Mathes EF, Gilliam AE. A four-year-old boy with fever, rash and arthritis. Semin Cutan Med Surg 2007; 26: 179-87. [ Links ]
10. Brucculeri M, Charlton M, Serur D. Serum sickness-like reaction associated with cefazolin. BMC Clinica Pharmacology 2006; 6: 1-3. [ Links ]
11. Vial T, Pont J, Pham E, Rabilloud M, Descotes J. Cefaclor-associated serum sickness-like disease: eight cases and review of the literature. Ann Pharmacotherapy 1992; 26: 910-4. [ Links ]
12. Lin B, Strehlow M. Serum-sickness-like reaction to amoxicilin. Ann Emerg Med 2007; 50: 350-9. [ Links ]
13. Barreia P, Gomes E. Doença do soro-like associada à administração de fármacos em idade pediátrica. Rev Port Imunoalergologia 2013; 21: 267-74. [ Links ]
14. Ferreira S, Ferreira S, Belo N, Estanqueiro P, Salgado M. Doença de Soro-like na criança. Saúde Infantil 2013; 35: 28-32. [ Links ]
15. Brockow K, Romano A, Blanca M, Ring J, Pichler W, Demoly P. General considerations for skin test procedures in the diagnosis of drug hypersensitivity. Allergy 2002; 57: 45-51. [ Links ]
16. Romano A , Blanca M, Torres MJ, Bircher A , Aberer W, Brockow K, et al. Diagnosis of nonimmediate reactions to β-lactam anti-biotics. Allergy 2004; 59: 1153-60. [ Links ]
17. Aberer W, Bircher A, Romano A, Blanca M, Campi P, Fernandez J, et al. Drug provocation testing in the diagnosis of drug hypersensitivity reactions: general considerations. Allergy 2003; 58: 854-63. [ Links ]
18. Demoly P, Adkinson NF, Brockow K, Castells M, Chiriac AM, Greenberger PA, et al. International Consensus on drug allergy. Allergy 2014; 69: 420-37. [ Links ]
19. Brockow K, Przybilla B, Aberer W, Bircher AJ, Brehler R, Dickel H, et al. Guideline for the diagnosis of drug hypersensitivity reactions. Allergo J Int. 2015; 24: 94-105. [ Links ]
Endereço para correspondência | Dirección para correspondencia | Correspondence
Francisca Martins
Department of Pediatrics
Unidade Local de Saúde do Alto Minho
Estrada de Santa Luzia
4904-858 Viana do Castelo
Email: franciscamartins8@hotmail.com
Received for publication: 17.01.2019
Accepted in revised form: 17.06.2019