Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Portuguese Journal of Nephrology & Hypertension
Print version ISSN 0872-0169
Port J Nephrol Hypert vol.34 no.1 Lisboa Mar. 2020
https://doi.org/10.32932/pjnh.2020.04.059
ORIGINAL ARTICLE
ABO-incompatible living donor kidney transplantation in Portugal
Catarina Isabel Ribeiro1,*, Natália Silva2,*, Jorge Malheiro3, Manuela Almeida3, Sofia Pedroso3, Maria La Salete Martins3, Leonídio Dias3, António Castro Henriques3, António Cabrita3
* Both authors contributed equally to this work
1 Serviço de Nefrologia – Centro Hospitalar de Vila Nova de Gaia/ Espinho
2 Serviço de Nefrologia – Centro Hospitalar de Tras‑Os‑Montes e Alto Douro
3 Serviço de Nefrologia/ Unidade de Transplantação Renal – Centro Hospitalar Universitário do Porto, Porto – Portugal
ABSTRACT
Introduction: ABO incompatibility was considered a barrier to kidney transplant. However, the shortage of available organs for transplantation and the excellent long‑term results further establish ABO‑incompatible (ABOi) as a safe and effective therapeutic strategy. The aim of the present study was to evaluate the outcomes of ABOi transplantation in terms of graft survival and function, rejection episodes and infections complications. Methods: The authors present a single‑center retrospective observational study in a unit with approximately 370 living donor kidney transplants registered. This study includes the analysis of 12 patients who underwent ABOi living donor kidney transplantation between November 2014 and July 2019. Desensitization protocol consisted of intravenous Rituximab 375mg/m2 single dose administration 2 weeks pretransplant. Tacrolimus and Mycophenolate Mofetil were started before transplantation one week and 48 hours respectively. Plasmapheresis was performed to remove anti‑A or B antibodies until their titers were <1:8 during the first post‑operative week and <1:16 at the second. All kidney recipients of both ABOi grafts received Basiliximab (20mg on days 0 and 4) as antibody induction therapy. Maintenance immunosuppression consisted of Tacrolimus, Mycophenolate Mofetil and corticosteroid. Results: A total of 12 patients were included in the study, 75% male; 43 years (IQR 31‑50). The most common blood group mismatch was A to O (n=4; 33%). In the first year, 2 of patients (25%) developed acute rejection. The follow‑up time was 17 months (IQR 7‑36). Five patients (42%) developed infectious complications. None patients developed cytomegalovirus or BK polyomavirus infections. At the end graft and patient survival were 100%. Conclusion: ABOi kidney transplantation has become a routine procedure. With this approach, about 30% of living donors who were refused in the past can now donate their kidneys and thereby significantly expand the living donor pool. The immunosuppressive protocol of this unit can be considered safe.
Keywords: ABO incompatible, Kidney Transplantation, Living Donor, Portugal
INTRODUCTION
Kidney transplantation is the treatment modality of chronic kidney disease G5D of Kidney Disease Improving Global Outcomes most associated with better survival rate and quality of life.1 The mean time on the waiting list for an available organ in Portugal is about five years and is growing every year. Given the shortage of deceased donors, efforts have been made to expand the living donor pool.2
The shortage of available organs for transplantation and the excellent long‑term results further establish ABOi as a safe and effective therapeutic strategy for the management of end‑stage renal disease patients.3,4
ABOi kidney transplantation is complex as the non‑removal of isoagglutinins in the recipient's circulation can result in early acute rejection by ABO antibodies.2,4 In the literature, acute antibody‑mediated rejection rates have been reported between 10% and 50%. With the introduction of new therapies, the ABO barrier for kidney transplantation has been breached.5‑7
Studies have also shown a high mortality rate in the incompatible ABO group mainly due to the increased risk of infections.8
Nevertheless, data from Japan demonstrating successful long‑term results of ABOi kidney transplantation combined with subsequent successful results from ABOi desensitization protocols in the United States have restored interest in this procedure.9,10 Nowadays, graft survival of ABOi kidney transplantation recipients matches those of ABO compatible recipients.6
In 2014 Oporto Hospital University Center started the ABOi kidney transplantation, using the recipient preconditioning protocols that had been used successfully in other European centers. In Portugal, the Oporto Hospital University Center is a pioneer in this type of transplant and the results of mismatched ABO donor/ recipient kidney transplants have not yet been reported.
MATERIAL AND METHODS
The authors present a single center retrospective observational study that included descriptive analysis of all patients undergoing ABOi living donor kidney transplantation in Portugal between 1 November 2014 and 31 July 2019. A total of 12 patients were transplanted. All these patients were admitted to the hospital 1 week before kidney transplant.
Desensitization protocol consisted of intravenous Rituximab 375mg/m2 single dose administration 2 weeks pretransplant. Tacrolimus (0.15mg/Kg/day) and Mycophenolate Mofetil (1g 12/12h) were started before transplantation one week and 48 hours respectively. The patients were admitted for isoagglutinin removal by plasmapheresis therapy. Before transplant, plasmapheresis was performed every other day (one plasma volume exchanged with 5% albumin) followed by 100mg/Kg of intravenous immunoglobulin. The final pretransplant and all post‑transplant Plasmapheresis sessions were performed with donor blood type fresh frozen plasma. The number of pretransplant plasmapheresis sessions was determined by the baseline of isoagglutinin IgG titer Isoagglutinin IgG titer was monitored on a daily basis and transplantation was performed once the titer reached ≤1:8. Intraoperatively, Methylprednisolone (500mg) and Basiliximab (20mg) were given as induction. Another dose of Basiliximab was repeated on the fourth postoperative day (POD). On the fifth POD, intravenous Methylprednisolone was slowly tapered to oral Prednisolone 20mg/day. Target Tacrolimus trough level was 8‑10ng/mL.
Patient follow‑up and the isoagglutinin titer measurement were performed twice weekly for the first month, weekly in the second and third month and monthly thereafter in the first year. During every visit, renal function tests including serum creatinine and hemogram were monitored. Human leukocyte antigen (HLA) antibodies and crossmatches were determined in accordance with the guidelines of the European Federation for Immunogenetics. Tacrolimus trough level target was 8‑10ng/mL during first three months, 7‑8ng/mL from 3 to 6 months, and 5‑7ng/mL thereafter. Prednisolone was tapered to 10mg by the end of 3 months and 5mg by the end of 6 months. Mycophenolate Mofetil was tapered to 500mg twice daily by 3 months.
Statistical data analysis was performed with SPSS® software (version 24). Continuous variables were described with median presentation (IQR 25 and IQR 75).
RESULTS
A total of 12 patients were included for the final analysis. The median follow‑up period was 17 months (IQR 7‑36).
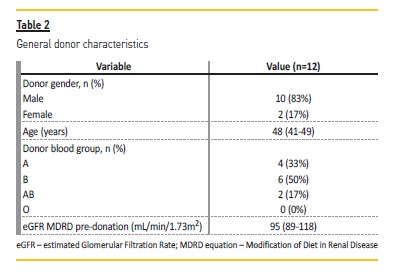
The characteristics of the ABOi kidney transplantation recipients and donors are summarized in Table 1 and 2, respectively. Short and long‑terms outcomes are described in Table 3.
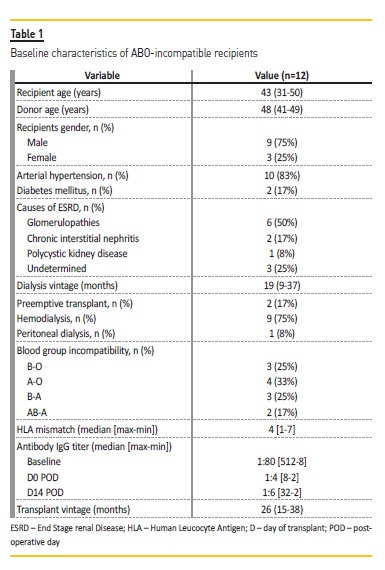
Median serum creatinine at this discharge, first month, first year and last evaluation was 1.48 (1.27‑1.64), 1.40 (1.22‑1.56), 1.58 (1.34‑1.91), 1.59 (1.12‑1.91) mg/dL, respectively. Delayed graft function occurred in 2 patients who recovered with normalized serum creatinine at discharge.
There were 2 patients (17%) of biopsy‑proven acute rejection (AR). One of the patients developed one episode of T cell mediated rejection (TCMR) Banff 2B that occurred in the immediate post‑transplant period. It was successfully treatment with anti‑thymocyte globulin (ATG) and Methylprednisolone. Within a month, the same patient was readmitted with acute graft disfunction, in the context of biopsy‑proven Acute Antibody Mediated Rejection (AAMR). Isoagglutinin IgG titers were persistently <1:8 and there were no detectable donor specific antibodies. He was successfully treated with plasmapheresis, immunoglobulin intravenous, Rituximab and corticotherapy. At 12 months after kidney transplantation, the patient underwent protocol biopsy. Histological findings were consistent with transplant glomerulopathy.
Serum creatinine at the time was 2.00mg/dL. The second patient evolved with delayed graft function 15th POD in the context of an AAMR. Isogglutinin IgG titer measurement was performed daily during the hospital stay and it was persistently <1:8 and there were no detectable donor specific antibodies. After treatment with plasmapheresis and corticoid he had a favorable outcome.
One patient with increasing levels of antibodies had stable renal function and protocol was maintained until the patient had a ratio less than 1:16 in the third week. This patient had no complications during follow‑up.
Ten patients (83%) developed mild leucopenia (2900‑3000/uL) and 8 (66%) moderate to severe anemia (6‑8g/dL). Six patients (50%) needed blood cell transfusion.
Five patients (42%) developed infectious complications during the follow‑up. Two (17%) developed pneumonia and other 2 (17%) had graft pyelonephritis with acute kidney injury. One patient (8%) was diagnosed with gastroenteritis. None of the patients developed cytomegalovirus or BK polyomavirus infections. At the end of follow‑up time, graft and patient survival were 100%.
DISCUSSION AND CONCLUSION
Especially in countries with long waiting lists for patients on maintenance dialysis, ABOi kidney transplantation constitutes an attractive alternative therapeutic option.1,11 During the last decade, the expansion of new, safe and potent immunosuppressors have enabled us to perform ABOi kidney transplantation in many centers worldwide.11
Patient survival was 100% in the current study. It was 98% in the study by Tyden et al. and 100% in the study by Flint et al. and Lipshutz et al.3,4,12 In a Korean study, Shin et al reported survival rates of patients at 1 and 3 years of 97% and 96% respectively.13
Death censored graft survival of ABOi transplant group in our study was comparable to that mentioned in other centers across the world.
Tyden et al., Flint et al. and Pankhurst et al. reported a graft survival of 97%, 100% and 90%, respectively.3,12,14
Although the outcomes of ABOi kidney transplantation recipients are quite favorable, these patients are at increased risk of early antibody‑mediated rejection and graft loss. In this study the authors report 3 episodes of AR (25%) in 2 patients. Two of these acute rejections were antibody mediated. Opelz et al. present a similar result of 16%. 6 On the other hand, Lipshutz et al. and Uchida et al. reported biopsy‑proven acute rejection of 11% and 32%, respectively.4,9 Most of the studies have reported 5% to 33% incidence of antibody‑mediated rejection in ABOi recipients.5,7 In our study, two ABOi recipients developed one episode of antibody‑mediated rejection each (17%). Only one developed TCMR (8%). A local available commercial kit for HLA antibodies and non‑HLA antigens was employed in both patients and none tested positive. However, recent findings opened up a new prospect in this field; the genomic collision may be a relevant underlying mechanism to consider in such cases.15
Infection rate was 42% in the present study. Hwang et al. also reported high rate of infection complications in ABOi kidney transplantation recipients (66%).5 Infection has been reported as the most common cause of death in previous studies. This appears to be because of the increased intensity of induction protocols and the subsequent immunosuppression needed for maintenance and to prevent graft rejection.8,11,16
Improved immunosuppression has significantly reduced the allograft rejection rate, although it can increase susceptibility to opportunistic infections. In this study of ABOi kidney transplantation recipients, the authors found low risks of infectious complications with excellent patient and renal graft survival. However, the small sample size and the short follow‑up period are important limitations of this study and conclusions have to be drawn with precaution.
In conclusion, ABOi kidney transplantation has become a routine procedure. By this approach, about 30% of living donors who were refused in the past can now donate their kidneys and thereby significantly expand the living donor pool.2 ABOi kidney transplantation provides an additional option for transplant with excellent results.
References
1. Kramer A, Pippias M, Noordzij M, Stel V, Andrusev A, Aparicio‑Madre M, et al. The European Renal Association ‑ European Dialysis and Transplant Association (ERA‑EDTA) Registry Annual Report 2016: a summary. Clin Kidney J. 2019;12(5):702‑720. [ Links ]
2. Christian M, Martin Z, Bernd D, Gerhard O, Caner S. ABO‑incompatible kidney transplantation. Front Immunol. 2017;8 234. [ Links ]
3. Flint S, Walker R, Hogan C, Haeusler M, Robertson A, Francis D, et al. Successful ABO‑incompatible kidney transplantation with antibody removal and standard immunosuppression. Am J Transplant. 2011;11:1016‑1024. [ Links ]
4. Lipshutz G, McGuire S, Zhu Q, Ziman A, Davis R, Goldfinger D, et al. ABO blood type‑incompatible kidney transplantation and access to organs. Arch Surg. 2011;146:453‑458. [ Links ]
5. Hwang J, Kim Y, Kim J, Chung B, Choi B, Yang C, et al. Comparative analysis of ABO‑incompatible living donor kidney transplantation with ABO‑compatible grafts: a single‑center experience in Korea. Transplant Proc. 2013;45:2931‑2936. [ Links ]
6. Opelz G, Morath C, Süsal C, Tran TH, Zeier M, Döhler B. Three‑year outcomes following 1420 ABO‑incompatible living‑donor kidney transplants performed after ABO antibody reduction: results from 101 centers. Transplantation. 2015;99:400‑404. [ Links ]
7. Uchida J, Kosoku A, Naganuma T, Tanaka T, Nakatani T. Latest insights on ABO‑incompatible living‑donor renal transplantation. Int J Urol. 2020 Jan;27(1):30‑38. [ Links ]
8. Habicht A, Broker V, Blume C, Lorenzen J, Schiffer M, Richter N, et al. Increase of infectious complications in ABO incompatible kidney transplant recipients a single center experience. Nephrol Dial Transplant. 2011;26:4124‑4131. [ Links ]
9. Uchida J, Kuwabara N, Machida Y, Iwai T, Naganuma T, Kumada N, et al. Excellent outcomes of ABO‑incompatible kidney transplantation: a single‑center experience. Transplant Proc. 2012;44:204‑209. [ Links ]
10. Mustian M, Cannon R, MacLennan P, Reed R, Shelton B, McWilliams D, et al. Landscape of ABO‑incompatible live donor kidney transplantation in the US. J Am Coll Surg. 2018;226(4):615‑621. [ Links ]
11. Christina M, Smaragdi M, George L, Chrysanthi S, Maria G, George Z, et al. Excellent long term patient and renal allograft survival after ABO‑incompatible kidney transplantation: experience of one center. World J Transplant. 2015;5(4):329‑337. [ Links ]
12. Tyden G, Donauer J, Wadström J, Kumlien G, Wilpert J, Nilsson T, et al. Implementation of a protocol for ABO‑incompatible kidney transplantation – a three‑center experience with 60 consecutive transplantations. Transplantation. 2007;83:1153‑1155. [ Links ]
13. Shin E, Kwon S, Yang W, Baeck C, Yu H, Cho H, et al. Long‑term outcomes of ABO‑incompatible living donor kidney transplantation: a comparative analysis. Transplantation Proc. 2015;47:1720‑1726. [ Links ]
14. Pankhurst L, Hudson A, Mumford L, Willicombe M, Galliford J, Shaw O, et al. The UK national registry of ABO and HLA antibody incompatible renal transplantation: pretransplant factors associated with outcome in 879 transplants. Transplant Direct. 2017;3:e181. [ Links ]
15. Steers N, Li Y, Drace Z, D'Addario J, Fischman C, Liu L, et al. Genomic Mismatch at LIMS1 Locus and Kidney Allograft Rejection. N Engl J Med. 2019;380(20):1918‑1928. [ Links ]
16. Okumi M, Kakuta Y, Unagami K, Takagi T, Lizuka J, Inui M, et al. Current protocols and outcomes of ABO‑incompatible kidney transplantation based on a single‑center experience. Transl Androl Urol. 2019;8(2):126‑133. [ Links ]
Catarina Isabel Ribeiro, MD
E‑mail: catarina.isabel.ribeiro@gmail.com
Natália Silva, MD
E‑mail: nataliasofia_1@hotmail.com
Nephrology Department, Centro Hospitalar de Vila Nova de Gaia – Espinho
Rua da Palmeira, nº 147 1º esquerdo, Laborim
4430‑163 Vila Nova de Gaia, Porto – Portugal
Disclosure of potential conflicts of interest:none declared
Received for publication: Dec 31, 2019
Accepted in revised form: Mar 28, 2020














