Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Portuguese Journal of Nephrology & Hypertension
Print version ISSN 0872-0169
Port J Nephrol Hypert vol.33 no.3 Lisboa Sept. 2019
https://doi.org/10.32932/pjnh.2019.10.033
REVIEW ARTICLE
Vitamin D – new insights into an old molecule
Cristina Jorge
Nephrology Department, Hospital de Santa Cruz – Centro Hospitalar de Lisboa Ocidental, Lisbon,Portugal
ABSTRACT
New data on vitamin D has emerged in the last fifteen years and continues to expand practically every day. It’s almost impossible to describe its full actions in a short article. In this review only a few aspects of this family of compounds are described, namely its endocrine pathway, and a few of its pleiotropic effects. Some of the known consequences of vitamin D deficiency are listed and special attention is given to its metabolism and the best way to supplement it, according to the author.
Keywords: Vitamin D, Bone, PTH, Immune system, Human disease
THE DISCOVERY OF VITAMIN D
McCollum et al inferred the existence of vitamin D in 1922, when they discovered that animals fed a low calcium diet grew appropriately if cod liver oil was added to their food. They assumed that a substance present in fat liver oil had anti-rachitic properties1. Steenbock et al found in 1924-1925 that the irradiation of ultraviolet (UV) light on animals as well as their foods could result in the activation of a lipid that had anti-rachitic properties1. In spite of this, the structure of vitamin D was only discovered later, in 1932, by Askew and colleagues, who isolated vitamin D2 after the UV light irradiation of ergosterol2.
Vitamin D3 was identified in 1936 by Windaus and Bock3. In 1977 Holick et al identified the process of synthesis of previtamin D3 from 7-dehydrocholesterol by the action of UV light on the skin4. In 1978, Esvelt and co-workers isolated and identified vitamin D3 by mass spectrometry5.
VITAMIN D METABOLISM
Vitamin D is either synthesized in the skin or absorbed in the diet. Skin synthesis is obtained by the action of UVB light (290-315 nm), which transforms 7-dehydrocholesterol into previtamin D3. This is turned into vitamin D3 or cholecalciferol by heat, entering the circulation.
Skin irradiation never leads to toxic levels of vitamin D, since continuing sunlight by itself destroys excess vitamin D6. The cutaneous synthesis of the vitamin is influenced by age, skin pigmentation, sunscreen use, time of the day, weather, season, latitude, altitude and air pollution7,8. As we get older, the amount of 7-dehydrocholesterol in the skin, and consequently our ability to produce vitamin D, diminishes6.
In response to UV light, white humans produce more vitamin D than melanodermic humans, since melanin absorbs UV light and constitutes a natural filter to it. Proper use of sunscreen of factor (SPF) 8 reduces vitamin D synthesis by 92.5% and SPF 15 by 99%6.
Vitamin D2 (ergocalciferol) comes from various plant species, such as mushrooms, after irradiation of UVB light. Vitamin D3, synthesized in the skin, is also present in several fatty fish, including salmon, mackerel and sardines. The amount of vitamin D in food is usually not enough for humans, and the main source of vitamin D comes from the skin, after sun irradiation6.Vitamin D2 or D3 absorbed by the diet is incorporated in chylomicrons and transported into the circulation.
Once in the circulation, vitamin D (either D2 or D3), which is transported by the vitamin D binding protein (VDBP), can be stored in fat tissue (and later released from it), or can pass through the liver, where it suffers hydroxylation by the action of 25 hydroxylase (the main enzyme being CYP2R1) and turns into 25 hydroxyvitamin D (25(OH) vitamin D), also known as calcidiol6. In fact, there seem to be other 25 hydroxylases apart from CYP2R1. However, this one in particular has remained stable through several vertebrate species and appears to be responsible for most of the 25 vitamin D hydroxylation9. Calcidiol also binds itself to VDBP and returns to the circulation afterwards. This first hydroxylation of vitamin D is an unregulated process10.
When passing through the kidney, the complex 25(OH)vitamin D/VDBP attaches itself to the megalin receptor of proximal tubule cells, is internalized and transformed into either 1,25 (OH)2 vitamin D (calcitriol) by the action of 1 alpha hydroxylase (now known as CYP27B1) or into 24,25 (OH)2 vitamin D (an inactive metabolite) by the action of CYP24A1 (24 hydroxylase). Calcidiol is the main form of circulating vitamin D in our organism and, due to its longer half-life (approximately 2-3 weeks) its blood level is thought to be indicative of the vitamin D status of an individual6,11. The half-life of vitamin D is indeed about 24 hours, and that of 1,25(OH)2 vitamin D is only a few hours11,12.
The molecular structure of vitamin D3, vitamin D2, 25(OH) vitamin D3 and 1,25 (OH)2 vitamin D3 is depicted in Fig. 1. These molecules are considered secosteroid hormones (steroids with a broken ring).
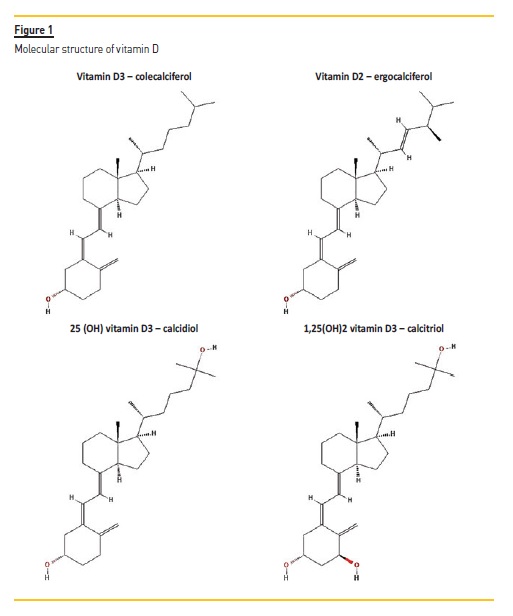
THE ENDOCRINE ROLE OF VITAMIN D
Calcitriol is the active form of vitamin D. It has the important task of controlling calcium and phosphorous levels and calcifying bone.
This is evidenced by rickets, which develop in children without adequate production of calcitriol (or with flaws in its metabolic machinery), and in adults by osteomalacia, a condition which causes a lack of mineralization of the osteoid matrix resulting in the accumulation of unmineralized osteoid tissue in the trabecular and cortical bone13.
Under normal conditions only the calcitriol that is produced in the kidney gets into circulation14. The classical mode of action of active vitamin D is depicted in Fig. 2.
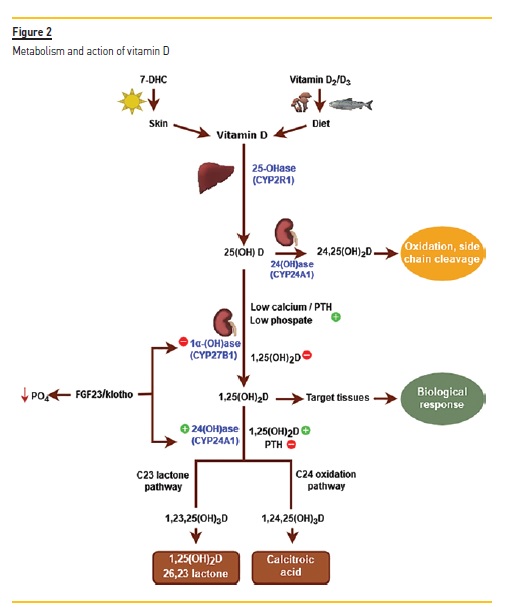
The renal synthesis of 1,25 (OH)2 vitamin D is tightly regulated and controlled by calcium (inhibition), the parathyroid hormone (PTH) (stimulus) and fibroblast growth factor-23 (FGF23) (inhibition). When serum calcium is low, PTH is released, which stimulates 1 alpha hydroxylase (CYP27B1) and consequently increases calcitriol synthesis. Calcitriol induces calcium absorption from the intestine (mainly through the transcellular pathway, but also through the paracellular pathway) and reabsorption of calcium (in conjunction with the PTH) from the distal tubule in the kidney and the mobilization of calcium from bone15,16.
PTH inhibits 24 hydroxylase (CYP24A1), which catabolises calcitriol to its inactive metabolites, and so limits the degradation of calcitriol.
Calcitriol, in turn, suppresses the production of PTH directly (at the transcription level) and indirectly (after increasing calcium levels and upregulating and increasing the calcium sensing receptor at the parathyroid gland). Also, calcitriol limits its own production by inhibiting CYP27B1 and promotes its own degradation by stimulating CYP24A116.
FGF23, which is produced by osteocytes, is a phosphaturic factor that acts by precluding phosphate absorption in the proximal tubule of the kidney. It is released in response to high phosphate levels and 1,25 (OH)2 vitamin D. FGF23 needs a cofactor (αKlotho), in order to interact with its receptor (FGFR) in the kidney. Klotho is a transmembrane protein that is highly abundant in the proximal and distal tubule17. The complex FGF23-Klotho suppresses 1 alpha hydroxylase (CYP27B1) and stimulates CYP24A1. As such, it acts in two ways: inhibiting the production of calcitriol and promoting its degradation16.
THE EFFECTS OF VITAMIN D ON BONE
Apart from influencing calcium levels, a more complex view of the actions of vitamin D in bone, was recently defended. Effectively, it appears that the action of vitamin D in bone depends on its target cell, as well as the extracellular calcium13,18. In order to understand it, it is convenient to remember that the receptor activator of NF-κB ligand (RANKL), which is produced by several cell types including osteocytes and osteoblasts, is a membrane protein that acts as an osteoclast differentiation and activation factor, after connecting to RANK in the osteoclast or its precursors19,20. Osteoprotegerin (OPG), a soluble glycoprotein that is highly expressed in osteoblastic lineage cells, (among others), binds to RANKL and therefore avoids its connection to its receptor RANK in the osteoclasts, inhibiting bone resorption20.
As such, the ratio RANKL/OPG in bone is a marker of bone resorption20. It is now known that bone formation is linked to the wingless (Wnt) signalling, which stimulates the osteoblastic lineage cells. One of its co-receptors is the lipoprotein receptor-related protein (LRP)-5. Returning to vitamin D, Goltzman describes in detail what is known in terms of the action of vitamin D in bone. So, in the presence of hypocalcemia, the consequent increase of 1,25(OH)2 vitamin D, acting through the VDR in immature osteoblasts, increases osteoclastogenesis by increasing the ratio RANKL/OPG, and therefore stimulating osteoclastic bone resorption, and reducing trabecular bone. On the other hand, in the presence of normal levels of 1,25 (OH)2 vitamin D (and in the absence of hypocalcemia), calcitriol, acting in mature osteoblasts or osteocytes, through their VDR, may mediate an increase in bone formation and a decrease in bone resorption simultaneously.
In fact, this last action is mediated by the decrease in the ratio RANKL/OPG in those cells. Furthermore, the bone formation seems to be mediated through the increased expression of (LRP)-5 of the Wnt pathway. Due to this, it is now believed that vitamin D may have either a catabolic or an anabolic effect on bone.
NON-CLASSICAL ACTIONS OF VITAMIN D
It is now widely accepted that the machinery for the metabolism of vitamin D is present in numerous cell types and tissues. This implies that vitamin D or 25(OH) vitamin D can get inside the cell and be hydroxylated to either 25(OH) vitamin D or 1,25(OH)2 vitamin D, and then destroyed to inactive metabolites inside the same cell. This intracellular active vitamin D (1,25(OH)2 vitamin D) acts locally, independently of the endocrine system. Until recently it was thought to exert its actions exclusively in the cell nucleus, activating or inactivating numerous genes. Nowadays, it is known to act also in the cytoplasm and to exert non-genomic actions, as we will see below.
When it comes to the availability of vitamin D, it is important to mention the work made with rats null for VDBP21. In these models, the only way vitamin D can get into the cell is by diffusion. And effectively, they show a normal growing if fed daily with vitamin D21.
As stressed by Hollis and Wagner, the main determinant of how long a vitamin D metabolite will stay in the circulation is its affinity for VDBP11. This affinity is higher for 25(OH) vitamin D, followed by vitamin D and smallest for 1,25 (OH)2 vitamin D, matching their half-lives. This affinity (or constant dissociation) also dictates the free circulating compound that is available to diffuse freely across cellular membranes.
In fact, it is highest for 1,25 (OH)2 vitamin, intermediate for vitamin D and smallest for 25(OH)vitamin D11. So, vitamin D (cholecalciferol) is more easily diffused through the cellular membrane than 25(OH) vitamin D. It is important to remember that, in the kidney, the megalincubilin endocytic system is responsible for the internalization of the complex 25 (OH) vitamin D/VDBP11. In the parathyroid gland, where megalin is also present, a similar process is likely in place. The same endocytic system also operates in the placenta11. In tissues where this system is not operative, the diffusion of these steroids becomes essential to their entry into the cell.
LESSONS FROM MATERNITY AND NEW DATA
Some information has become available from human observations concerning pregnancy and breast-feeding. In pregnant women the levels of 1,25(OH)2 vitamin D are much higher than in normal, nonpregnant adults. In spite of this, there is no hypercalcemia during pregnancy. Therefore, the levels of 1,25(OH)2 vitamin D during pregnancy are uncoupled to the calcium homeostasis and are largely dependent on the availability of the substrate, 25(OH) vitamin D22. It is believed that the role of 1,25(OH)2 vitamin D during pregnancy is mainly related to immune modulation and maternal tolerance towards the fetus22. An association between preeclampsia and low levels of 1,25 (OH) vitamin D is known to exist in the mother23. Recently, 2 small randomized controlled studies found a beneficial effect of vitamin D supplementation in the incidence of this entity24,25. However, a mendelian study with thousands of pregnant women did not find a clear causal effect between preeclampsia or hypertension during pregnancy with vitamin D deficiency26. This condition, preeclampsia, which is defined as the existence of de novo hypertension accompanied by proteinuria after 20 weeks’ gestation, is associated with vasculitis and inflammation27. It is viewed as an endothelial dysfunction28.
Recently, as elegantly shown by Gibson et al on human microvascular endothelial cells, vitamin D was found to exert an important role in the stabilization of intercellular connections on the endothelium.
Moreover, this action was accomplished either by vitamin D, 25(OH) vitamin D or 1,25(OH)2 vitamin D, but the most potent molecule to perform this task was proved to be the first one29. This was a new finding: until then, it was believed that vitamin D per se had no important role to play in the human body. As also shown by the authors, this action of vitamin D happened within minutes, and they concluded that it was non-genomic, seemingly independent from the VDR. This action of vitamin D could explain the association of vitamin D deficiency with numerous human pathologies like hypertension, cardiovascular disease and overall mortality29. In terms of importance of the sterol vitamin D, Hollis and Wagner have defended its crucial role for some time, coming to that conclusion after conducting various experiments with lactating women. First, Greer et al published in 1984 an article analysing the vitamin D content of human milk and found that when mothers were irradiated with UVB light, their serum content of both vitamin D and 25(OH) vitamin D increased, as expected. In spite of this, only the vitamin D in the milk rose after UVB exposure, without change in the amount of 25(OH) vitamin D30. So, the transfer of vitamin D from serum to human milk is much more efficient than the transfer of 25 (OH) vitamin D. Hollis and Wagner, who have been working in this area for decades, debated the topic in depth in a 2013 article where they analysed the importance of vitamin D itself11. They believe that, rather than the bonding of vitamin D to VDBP, it is the higher free serum concentration of vitamin D that permits the easier free diffusion of vitamin D across the mammary gland to the milk, rather 25(OH) vitamin D. So, in terms of breast-feeding, and in order to supply the baby with enough vitamin D, the mother should be replenished with vitamin D. If sun exposure is not adequate or possible, then a daily supplement of at least 6000 UI of vitamin D should be administered to the mother, according to the same authors. It is noteworthy to remember that, during pregnancy, the passage of vitamin D to the fetus is carried out mainly by calcidiol. It is thought that this happens because 25(OH) vitamin D has high affinity for VDBP and, in the placenta, the megalin-cubilin endocytic system is operative11.
As discussed above, it has become evident that some of the observed effects of vitamin D were too fast to be considered at the gene level. Previously it was thought that all the vitamin D actions resulted from the coupling of calcitriol to VDR, which belongs to a sub-family of nuclear receptors. By forming a heterodimer with retinoic X receptor (RXR), and VDR, 1,25(OH)2 vitamin D induces activation of VDR, which connects to a vitamin D response element (VDRE) and initiates transcription of a variety of genes. These depend on the cell type, maturation status of the cell, among other factors31. It is estimated that vitamin D may interact with or influence up to 2000 genes7.
EFFECTS OF VITAMIN D ON THE IMMUNE SYSTEM
Vitamin D has an important role in both innate and adaptive immunity. Immune system cells, such as monocytes and dendritic cells, express the VDR and possess the machinery necessary to convert 25 (OH) vitamin D to 1,25 (OH)2 vitamin D and to metabolize it to inactive vitamin D metabolites. As extensively reviewed by Hewison, vitamin D induces bacterial killing of Mycobacterium tuberculosis by monocytes, through a VDR synthesis of cathelicidin and beta-defensin 2.
These peptides have bactericidal properties. The intracellular synthesis of 1,25(OH)2 vitamin D also induces autophagy, a cytoplasmic process that enhances bacterial killing32. T and B Lymphocytes, although with a very low expression in resting state, show increased levels of VDR upon cellular activation. As such, in sites of inflammation it is possible to convert 25 (OH) vitamin D to its active form at a cellular level33.
Peelen et al in 2011, published an article on the effects of vitamin D in each cell of the immune system, where the reader can find an in depth and detailed description of this subject33. In general, the effects of vitamin D on the immune system appear to go into an immunomodulatory and tolerogenic action. Specifically, vitamin D induces a shift in the balance T helper 1 / T helper 2 cells towards a predominant T helper 2 cells and decrease of T helper 17 cells response33. Recently various studies performed with human cells and even humans, have confirmed those assumptions. Drozdenko et al supplemented 25 people with different doses of cholecalciferol (between 2000 until 8000 UI per day) for 12 weeks in order to attain blood levels of 25 (OH) vitamin D of at least 44 ng/mL (or 110 nmol/L). Those individuals were compared to a control group of 18 people, which did not receive vitamin D34. They found that supplemented people showed an increased frequency of CD38 in peripheral B Lymphocytes and that this happened after 25(OH) vitamin D blood concentrations of approximately 28 ng/mL (69 nmol/L). They also noticed a decrease in IFN gamma and IL-17 secreting T cells after vitamin D supplementation and concluded that this happened for 25(OH) vitamin D concentrations of at least 28 ng/mL (70 nmol/L). They found no differences in immunoglobulin concentration or in IL-10 producing T cells. Prietl et al showed in 2010, in an uncontrolled pilot study performed on 46 apparently healthy adults, an increase in circulating regulatory T cells (CD4+CD25++FOXP3+ cells with low or absent expression of CD127) at 4 and 8 weeks after intramuscular supplementation with 140 000 UI cholecalciferol at baseline and four weeks later. They also observed growing of the mean serum levels of 25 (OH) vitamin D at 4 and 8 weeks (the duration of the study), from insufficiency (23.9 ± 12.9 ng/mL) to 58.0 ± 15.1 ng/mL at 8 weeks35. More recently, it was recorded in a community study involving 77 infants and children up to 12 years old, with community acquired pneumonia, that the patients with vitamin D deficiency had lower peripheral lymphocytes, less CD19 Lymphocytes and higher neutrophil counts than patients with vitamin D sufficiency36. The immunomodulatory effects of vitamin D have been argued to explain the action of vitamin D in several diseases like autoimmune disorders, response to infections or cancer7.
VITAMIN D AND HUMAN DISEASE
Apart from rickets, a disease that has been known for decades (causally associated with vitamin D deficiency), numerous pathologies have been linked to vitamin D in the recent years.
There are several epidemiologic studies relating vitamin D deficiency with the cardiovascular disease, cancer, immune mediated diseases, neuroendocrine diseases and infectious conditions, among others7.
Studies with animals confirmed that vitamin D influences the cardiovascular system. Indeed VDR or CYP27B1 null mice have increased renin and angiotensin II, are hypertensive and have left ventricular hypertrophy (LVH)37,38. Low serum levels of vitamin D are associated with increased PTH levels39. BrouliK et al showed, back in 1986, that PTH infusion in humans was found to cause increased renin activity as well40. In spite of this, in a group of 17 humans (6 to 36 year-old) with Hereditary Vitamin D–Resistance Rickets (HVDRR), the human disease to which the VDR null mice corresponds to, no increased renin activity, nor hypertension or LVH was found, at least until the age of 36. Although these patients had both high 1,25 (OH)2 vitamin D and PTH serum levels41. Despite this, a prospective human study that involved 3296 patients submitted to coronary angiography, an inverse association between 25 (OH) vitamin D and 1,25(OH)2 vitamin D with plasma renin and angiotensin 2 concentrations was found42.
So, even in human studies into vitamin D, the results are not consistent. Another way vitamin D seems to influence the cardiovascular system is through its action in the endothelium. Lower 25 (OH) vitamin D levels have been correlated with increased arterial stiffness and endothelial dysfunction in a population of 554 apparently healthy volunteers aged 20 to 79 years, in a cross sectional and observational study performed by Al Mheid et al43. One possible mechanism for this vitamin D mediated action might be through interference with nitric oxide levels44,45, by modulating inflammation45 or even by direct actions of vitamin D in the endothelial cells29,45. Further, vitamin D has been implicated in the process of vascular calcification. Either deficient or excess vitamin D levels have been associated with the active process of vascular calcification. Although the exact mechanisms to explain these outcomes are yet to be fully understood, a recent theory considers that there may be a biphasic response to vitamin D in the vasculature, and it may be related not only to serum levels but also to local regulators and intracellular metabolic pathways of vitamin D46.
Also, vitamin D deficiency has been associated to mortality. Observational studies and meta-analysis of observational studies have persistently shown low serum levels of 25(OH) vitamin D and increased overall mortality risk, sometimes with cardiovascular death or cancer related death47-52. In 2017, Martin Gaksch et al published a meta-analysis of individual participant data, comprising a total of 26916 people that were followed for a median period of 10.5 years.
The 25 (OH) vitamin D measurements were performed according to the certified liquid chromatography–tandem mass spectrometry method. They found an increasing mortality risk for 25 (OH) vitamin D levels less than 20 ng/mL52. One of the most recent populationbased studies was conducted in Olmsted County, Minnesota (USA) and included 11022 individuals, of whom 723 died after a median follow-up of 4.8 years. This population had a 25(OH) vitamin D mean (±SD) level of 30 ± 12.9 ng/mL. White people with 25 (OH) vitamin D less than 20 ng/mL had greater all-cause mortality than those with higher 25 (OH) vitamin D levels. In patients of other races/ethnicities this association between low levels of 25 (OH) vitamin D and mortality was not found53. In spite of an inverse association between vitamin D and mortality being consensual, the causality cannot be defined with these studies. Naturally, sicker individuals may have lower vitamin D levels as a consequence of their disease.
Mendelian randomization studies try to find a genetically defined characteristic with a specific outcome, and therefore can be useful in the search for causality. Afzal et al reported in 2014 a mendelian randomization study on alleles of genes implicated on the synthesis of 25 (OH) vitamin D (in the skin, from 7 dehydrocolesterol-DHCR7 and in the first hydroxylation in the liver-CYP2R1) in 95766 people of Denmark54. They concluded that genetically determined low levels of vitamin D were associated with increased overall and cancer mortality.
They did not find a causal relationship with cardiovascular mortality and hypothesized that this association in previous studies could be due to confounding. Recently a second mendelian randomization study analysed single nucleotide polymorphisms on the pathway of vitamin D synthesis and mortality in 10501 individuals from Iceland, Germany and Norway55. The 25 (OH) vitamin D evaluation was performed under the Vitamin D Standardization Program, to ensure all samples were analysed according with the certified liquid chromatography–tandem mass spectrometry method. The authors of this study concluded that there was an increase in mortality for 25(OH) vitamin D levels less than 16 ng/mL. Lastly, a metaanalysis recently published, which analysed 84 articles (from 2006 until 2018), and included 57 studies comprising mainly elderly people and with a follow-up that varied from 1.7 to 37 years, concluded that the majority of the studies found an inverse relationship between vitamin D levels and mortality56. In fact, there was a progressively lower mortality with higher levels of vitamin D up to a certain threshold beyond which there was no survival benefit. They considered that this relationship was mainly with mortality due to cancer and respiratory diseases and was much weaker with cardiovascular disease. In these last reports, a J or U-shaped curve between vitamin D and mortality was not noticed. Previous studies that found such a U-shape relationship47,57,58 were probably tainted with confounding or included people that were supplemented with vitamin D too late in the course of the disease59,60.
Despite all this knowledge, there is not, until now, a supplementation study with vitamin D that proved its ability to decrease mortality or cure diseases. The defenders of vitamin D argue that this is because of the varying doses and mode of administration of vitamin D, the short duration of the studies, the lack of quality of most studies (not randomized controlled studies), and so on. Surely, one should not expect that a study that lasts weeks or even months should show significant results in such a short period. Apart from this, and since mortality seems to be related with deficient 25(OH) vitamin D levels, it is reasonable to argue that vitamin D supplementation studies should be directed to people with low or very low levels of 25 (OH) vitamin D levels, in order to show a clear benefit from supplementation. Given the fact that most supplementation studies supplement everyone included, independently from the basal vitamin D levels, the net results of these studies may be diluted with people in whom supplementation does not bring any additional benefit. In this regard, Robert Heaney wrote guidelines to assist in the design, performance and interpretation of studies concerning nutrients, in which vitamin D can be included61.
VITAMIN D IN CKD – RECOMMENDATIONS
Active vitamin D and its analogs have long been used in chronic kidney disease (CKD) patients to control secondary hyperparathyroidism.
The current guidelines concerning vitamin D supplementation in CKD patients, issued by the KDIGO group, advise the use of native vitamin D in these patients just as in the general population, in order to correct vitamin D deficiency or insufficiency62. These guidelines also recommend caution when using active vitamin D in patients not on dialysis, due to the risk of hypercalcemia. Specifically, they recommend the use of either active vitamin D or active vitamin D analogs only in patients with CKD stages 4 to 5 with severe or progressive secondary hyperparathyroidism. In fact, studies with vitamin D analogs in CKD patients not on dialysis, such as the Primo study and the Opera study, in which paricalcitol was compared to placebo, respectively for 48 and 52 weeks, including about 260 patients, most with secondary hyperparathyroidism, and all with mild to moderate LVH, showed no benefit in the use of paricalcitol to reduce LVH or modify diastolic function63,64. Despite reducing PTH, more patients in the paricalcitol groups showed hypercalcemia in respect to placebo (22.6 % versus 0.9 % and 43.3% versus 3.3%) in both studies. The same guidelines refer to meta-analyses in which calcitriol and active vitamin D analogs were used which also showed increased hypercalcemia65,66.
On the other hand, the use of native vitamin D (cholecalciferol or ergocalciferol) in CKD patients has been found to be generally safe, not causing hypercalcemia nor hyperphosphatemia. Moreover, in CKD stages 3 through 4 it decreased (although modestly) PTH67.
In the 2017 paediatric guidelines for CKD, it is recommended to supplement children with CKD stages 2 to 5D to maintain 25 (OH) vitamin D levels over 30 ng/mL and to control secondary hyperparathyroidism, with either cholecalciferol or ergocalciferol68. If needed, the use of active vitamin D in order to control secondary hyperparathyroidism should be the least amount possible in order to diminish PTH and to maintain normocalcemia. According to the authors, there is no advantage in using one type of active vitamin D analog over the other69.
HOW AND HOW MUCH TO SUPPLEMENT WITH NATIVE VITAMIN D
In 2011, the Endocrine Society (ES) issued guidelines on the evaluation and treatment of vitamin D deficiency. The expert panel, after reviewing available data, defined vitamin D deficiency as a 25(OH) vitamin D level lower than 20 ng/mL, insufficiency a level between 21-29 ng/mL and sufficiency a level of at least 30 ng/mL and they considered safe a level up to 100 ng/mL70. In fact, as it was shown in a population of 1500 postmenopausal women in the United States, regarding the relation between PTH and 25 (OH) vitamin D, the PTH increases gradually with levels of 25(OH) vitamin D lower than 30 ng/mL71. The previously mentioned definitions were also adopted by other American and international associations72.
Since the prevalence of vitamin D deficiency is so frequent and seems to cause damage, and also given that its supplementation has shown to be safe, the question of why to supplement has a clear answer: within the physiologic limits, it does not harm, and might do some good
However, a harder question to answer is how and how much
First, supplementing with either ergocalciferol or cholecalciferol seems to cause similar effects in the long term, due to the fact that when chronically supplementing with each one, the levels of 25 (OH) vitamin D rise in a similar way73,74. A study compared the amount of 1,25 (OH)2 vitamin D produced by the kidney after 11 weeks of supplementation and found that in response to taking 1000 IU of ergocalciferol per day, there was production of 1,25 (OH)2 vitamin D2 and the amount of 1,25 (OH)2 vitamin D3 decreased accordingly, so that the total 1,25 (OH)2 vitamin D was kept constant. In response to the supplementation of 1000 IU of cholecalciferol per day, the kidney produced 1,25 (OH)2 vitamin D3. As such, the amount of total 1,25 (OH)2 vitamin D was similar, and (unsurprisingly) did not change, since the level of 1,25 (OH)2 vitamin D is tightly regulated by the endocrine system. Noteworthy, the amount of 25 (OH) vitamin D2 or D3 rose comparably with each type of vitamin D74.
The ES divides their amount recommendation into the daily allowance, i.e. the minimum requirement per day, and the tolerable upper limit (UL). Although to most people (from infants to adults) the minimum recommended dose varies between 400 – 800 IU per day, in order to attain the sufficient 25 (OH) vitamin D (over 30 ng/mL) blood level, most people will need at least 1500-2000 IU per day70. The ES does not specify the regularity of administration (daily, weekly, monthly or even three times a year), necessary to obtain effective levels of 25(OH) vitamin D. From the previous discussion in this article we can assume that, in terms of vitamin D levels, there is a difference between receiving it daily or three times per week or monthly. So in my opinion, if possible, and in order to maintain stable levels of not only 25 (OH) vitamin D, but also vitamin D, administration should ideally be daily (or maybe 3 times per week, as we have been doing in hemodialysis patients)75. In our report we used a liquid formulation of vitamin D3 (vigantol®), which contains 667 IU of vitamin D3 per drop.
FINAL REMARKS
There is still a lot we don’t know about vitamin D. Its main role seems to be within mineral and bone metabolism, as evidenced by diseases like rickets in children and osteomalacia in adults. Nowadays, it is known that vitamin D and/or its metabolites have numerous pleiotropic effects. Epidemiologic studies have associated low vitamin D levels with several diseases and mortality. In spite of that, to date, no randomized controlled study has shown to reduce mortality or improve significant outcomes in the general population, but we cannot forget those studies have limitations, as explained before. Since it seems to do no harm, supplementing deficient people with physiological doses of nutritional vitamin D appears to be safe and may be beneficial. This might be particularly important in specific populations like children, pregnant and breastfeeding women, those in menopause, the elderly and people with CKD.
References
1. DeLuca HF. History of the discovery of vitamin D and its active metabolites. Bonekey Rep. 2014;3 (July 2013): 1–8. [ Links ]
2. Askew F, Bourdillon R, Bruce H, Callow R, Philpot J, Webster T. Crystalline vitamin D. Proc R Soc Lond B. 1932; 109: 488–506. [ Links ]
3. Windaus A, Bock F. Über das Provitamin aus dem Sterin der Schweineschwarte. Hoppe Seylers Z Physiol Chem. 1936; 245 (3–4): 168–70. [ Links ]
4. Holick MF, Frommer JE, McNeill SC, Richtand NM, Henley JW, Potts JT. Photometabolism of 7-dehydrocholesterol to previtamin D3 in skin. Biochem Biophys Res Commun. 1977; 76 (1): 107–14. [ Links ]
5. Esvelt RP, Schnoes HK, DeLuca HF. Vitamin D3 from rat skins irradiated in vitro with ultraviolet light. Arch Biochem Biophys. 1978; 188 (2): 282–6. [ Links ]
6. Holick MF. Vitamin D Deficiency. N Engl J Med. 2007; 357 (3): 266–81. [ Links ]
7. Hossein-Nezhad A, Holick MF. Vitamin D for health: A global perspective. Mayo Clin Proc. 2013; 88 (7): 720–55. [ Links ]
8. Webb AR. Who, what, where and when-influences on cutaneous vitamin D synthesis. Prog Biophys Mol Biol. 2006; 92 (1): 17–25. [ Links ]
9. DeLuca HF, Zhu JG, Kaufmann M, Jones G, Ochalek JT. CYP2R1 is a major, but not exclusive, contributor to 25-hydroxyvitamin D production in vivo. Proc Natl Acad Sci. 2013; 110 (39): 15650–5. [ Links ]
10. Jones G. Pharmacokinetics of vitamin D toxicity. American Journal of Clinical Nutrition. 2008; 88 (2): 582S–586S. [ Links ]
11. Hollis BW, Wagner CL. The role of the parent compound vitamin D with respect to metabolism and function: Why clinical dose intervals can affect clinical outcomes. J Clin Endocrinol Metab. 2013; 98 (12): 4619–28. [ Links ]
12. Smith J, Goodman D. The turnover and transport of vitamin D and of a polar metabolite with the properties of 25-hydroxycholecalciferol in human plasma. J Clin Invest. 1971; 50 (10): 2159–67. [ Links ]
13. Goltzman D. Functions of vitamin D in bone. Histochem Cell Biol. 2018; 149 (4): 305–12. [ Links ]
14. Bouillon R, Marcocci C, Carmeliet G, Bikle D, White JH, Dawson-Hughes B, et al. Skeletal and extra-skeletal actions of vitamin D: Current evidence and outstanding questions. Endocrine Reviews. 2019; 40 (4): 1109–51. [ Links ]
15. Gil Á, Plaza-Diaz J, Mesa MD. Vitamin D: Classic and novel actions. Ann Nutr Metab. 2018; 72:87–95. [ Links ]
16. Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic rffects. Physiol Rev. 2016; 96 (1): 365–408. [ Links ]
17. Erben RG, Andrukhova O. FGF23-Klotho signaling axis in the kidney. Bone. 2017; 100: 62–8. [ Links ]
18. van Driel M, van Leeuwen JPTM. Vitamin D endocrinology of bone mineralization. Mol Cell Endocrinol. 2017; 453 (September): 46–51. [ Links ]
19. Xiong J, O’Brien CA. Osteocyte RANKL : New insights into the control of bone remodeling. J Bone Miner Res. 2012; 27 (3): 499–505.
20. Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther. 2007; 9 (1): S1. [ Links ]
21. Safadi FF, Thornton P, Magiera H, Hollis B, Gentile M, Haddad JG., et al. Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. J Clin Invest. 1999; 103 (2): 239–51. [ Links ]
22. Hollis BW, Wagner CL. New insights into the Vitamin D requirements during pregnancy. Bone Res. 2017; 5: 17030. [ Links ]
23. Bodnar LM, Simhan HN, Catov JM, Roberts JM, Platt RW, Diesel JC, et al. Maternal vitamin D status and the risk of mild and severe preeclampsia. Epidemiology. 2014; 25 (2): 207–14. [ Links ]
24. Sablok A, Batra A, Thariani K, Batra A, Bharti R, Aggarwal AR, et al. Supplementation of Vitamin D in pregnancy and its correlation with feto-maternal outcome. Clin Endocrinol. 2015 ;83 (4): 536–41. [ Links ]
25. Behjat Sasan S, Zandvakili F, Soufizadeh N, Baybordi E. The effects of vitamin D supplement on prevention of recurrence of preeclampsia in pregnant women with a history of preeclampsia. Obstet Gynecol Int. 2017; 2017: 1–5. [ Links ]
26. Magnus MC, Miliku K, Bauer A, Engel SM, Felix JF, Jaddoe VWV, et al. Vitamin D and risk of pregnancy-related hypertensive disorders: mendelian randomization study. Obstet Gynecol Surv. 2018; 73 (11): 617–9. [ Links ]
27. American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Obstet Gynecol. 2013; 122 (5): 1122–31. [ Links ]
28. Lim K, Steinberg G. Preeclampsia. emedicine.medscape.com. 2018 [cited 2018 Nov 29]. Available at https://emedicine.medscape.com/article/1476919
29. Gibson CC, Davis CT, Zhu W, Bowman-Kirigin JA, Walker AE, Tai Z, et al. Dietary vitamin D and its metabolites non-genomically stabilize the endothelium. PLoS One. 2015; 10 (10): 1–15. [ Links ]
30. Greer F, Hollis B, Cripps D, Tsang R. Effects of maternal ultraviolet B irradiation on vitamin D content of human milk. Clin Lab Obs. 1984; 105 (3): 431–3. [ Links ]
31. Chin WW, Burris TP, Buck DW, Houck KA, Nagpal S, Khalifa B, et al. Retinoid X receptor is a nonsilent major contributor to vitamin D receptor-mediated transcriptional activation. Mol Endocrinol. 2003; 17 (11): 2320–8. [ Links ]
32. Hewison M. Vitamin D and innate and adaptive immunity. Vitamins and Hormones. 1st edition. Elsevier Inc. 2011; 86: 23–62. [ Links ]
33. Peelen E, Knippenberg S, Muris A, Thewissen M, Smolders J, Willem J, et al. Effects of vitamin D on the peripheral adaptive immune system: A review. Autoimmun Rev. 2011; 10 (12): 733–43. [ Links ]
34. Drozdenko G, Heine G, Worm M. Oral vitamin D increases the frequencies of CD38+ human B cells and ameliorates IL-17-producing T cells. Exp Dermatol. 2014; 23 (2): 107–12. [ Links ]
35. Prietl B, Pilz S, Wolf M, Tomaschitz A, Obermayer-Pietsch B, Graninger W, et al. Vitamin D supplementation and regulatory T Cells in apparently healthy subjects: Vitamin D treatment for autoimmune diseases? Isr Med Assoc J. 2010; 12 (3): 136–9. [ Links ]
36. Huang Y, Fu L, Yang Y. Age-related vitamin D deficiency is associated with the immune response in children with community-acquired pneumonia. J Nutr Sci Vitaminol. 2017; 63 (1): 1–7. [ Links ]
37. Li YC, Kong J, Wei M, Chen Z, Liu SQ, Cao L. 1 , 25-Dihydroxyvitamin D 3 is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002; 110 (2): 229–38. [ Links ]
38. Zhou C, Lu F, Cao K, Xu D, Goltzman D, Miao D. Calcium-independent and 1,25(OH)2D3-dependent regulation
of the renin-angiotensin system in 1α-hydroxylase knockout mice. Kidney Int. 2008; 74 (2): 170–9.
39. Lips P, Van Schoor NM. The effect of vitamin D on bone and osteoporosis. Best Pract Res Clin Endocrinol Metab. 2011; 25 (4): 585–91. [ Links ]
40. Broulik PD, Horky K, Pacovsky V. Effect of parathyroid hormone on plasma renin activity in humans. Horm Metab Res. 1986; 18 (7): 490–2. [ Links ]
41. Tiosano D, Schwartz Y, Braver Y, Hadash A, Gepstein V, Weisman Y, et al. The renin-angiotensin system, blood pressure, and heart structure in patients with hereditary vitamin D-resistance rickets (HVDRR). J Bone Miner Res. 2011; 26 (9): 2252–60. [ Links ]
42. Tomaschitz A, Pilz S, Ritz E, Grammer T, Drechsler C, Boehm BO, et al. Independent association between 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and the renin–angiotensin system. Clin Chim Acta. 2010; 411 (17–18): 1354–60. [ Links ]
43. Al Mheid I, Patel R, Murrow J, Morris A, Rahman A, Fike L, et al. Vitamin D status is associated with arterial stiffness and vascular dysfunction in healthy humans. J Am Coll Cardiol. 2011; 58 (2): 186–92. [ Links ]
45. Kassi E, Adamopoulos C, Basdra EK, Papavassiliou AG. Role of vitamin D in atherosclerosis. Circulation. 2013; 128 (23): 2517–31. [ Links ]
46. Wang J, Zhou JJ, Robertson GR, Lee VW. Vitamin D in vascular calcification: A double-edged sword? Nutrients. 2018; 10 (5): 1–17. [ Links ]
47. Melamed M, Michos E, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008; 168 (15): 1629–37. [ Links ]
48. Melamed M TR. Vitamin D therapy in chronic kidney disease and end stage renal disease. Clin J Am Soc Nephrol. 2012; 7 (2): 8–65. [ Links ]
49. Zittermann A, Iodice S, Pilz S, Grant WB, Bagnardi V and Gandini S. Vitamin D deficiency and mortality risk in the general population. Am J Clin Nutr. 2012; 95 (1): 91–100. [ Links ]
50. Garland CF, Kim JJ, Mohr SB, Gorham ED, Grant WB, Gorham ED, et al. Meta-analysis of all-cause mortality according to serum 25-hydroxyvitamin D. Am J Public Health. 2014; 104 (8): e43–50. [ Links ]
51. Schöttker B, Jorde R, Peasey A, Thorand B, Jansen EHJM, De Groot L, et al. Vitamin D and mortality: Meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ. 2014; 348 (June): 1–15. [ Links ]
52. Gaksch M, Jorde R, Grimnes G, Joakimsen R, Schirmer H, Wilsgaard T, et al. Vitamin D and mortality: Individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PLoS One. 2017; 12 (2): e0170791. [ Links ]
53. Dudenkov D V., Mara KC, Petterson TM, Maxson JA, Thacher TD. Serum 25-hydroxyvitamin D values and risk of all-cause and cause-specific mortality: A population-based cohort study. Mayo Clin Proc. 2018; 93 (6): 721–30. [ Links ]
54. Afzal S, Brøndum-Jacobsen P, Bojesen SE, Nordestgaard BG. Genetically low vitamin D concentrations and increased mortality: Mendelian randomisation analysis in three large cohorts. BMJ. 2014; 349 (November): 1–12. [ Links ]
55. Aspelund T, Grübler MR, Smith A V., Gudmundsson EF, Keppel M, Cotch MF, et al. Effect of genetically low 25-hydroxyvitamin D on mortality risk: Mendelian randomization analysis in 3 large european cohorts. Nutrients. 2019; 11 (1): 74. [ Links ]
56. Heath AK, Kim IY, Hodge AM, English DR, Muller DC. Vitamin D status and mortality: A systematic review of observational studies. Int J Environ Res Public Health. 2019; 16 (3): 383. [ Links ]
57. Michaelsson K, Baron JA, Snellman G, Gedeborg R, Byberg L, Sundstro J, et al. Plasma vitamin D and mortality in older men: A community-based prospective cohort study. Am J Clin Nutr. 2010;92: 841–8. [ Links ]
58. Durup D, Jørgensen HL, Christensen J, Schwarz P, Heegaard AM, Lind B. A reverse J-shaped association of all-cause mortality with serum 25-hydroxyvitamin D in general practice: The CopD study. J Clin Endocrinol Metab. 2012; 97 (8): 2644–52. [ Links ]
59. Annweiler C, Garland CF, Karras SN, Juzeniene A, Bischoff-Ferrari HA, Boucher BJ, et al. Do studies reporting U’-shaped serum 25-hydroxyvitamin D–health outcome relationships reflect adverse effects? Dermatoendocrinol. 2016; 8 (1): e1187349.
60. Pilz S, Grübler M, Gaksch M, Schwetz V, Trummer C, Hartaigh BÓ, et al. Vitamin D and mortality. Anticancer Res. 2016; 36 (3): 1379–87. [ Links ]
61. Heaney RP. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr Rev. 2014; 72 (1): 48–54. [ Links ]
62. KDIGO CKD-MBD Update Work Group. Kidney Disease: Improving Global Outcomes (KDIGO) CKDMBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD). Kidney Int S. Kidney Int Suppl. 2017; 7(3): e1. [ Links ]
63. Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease. Jama. 2012; 307 (7): 674. [ Links ]
64. Wang AY-M, Fang F, Chan J, Wen Y-Y, Qing S, Chan IH-S, et al. Effect of paricalcitol on left ventricular mass and function in CKDThe OPERA trial. J Am Soc Nephrol. 2013; 25 (1): 175–86. [ Links ]
65. Xu L, Wan X, Huang Z, Zeng F, Wei G, Fang D, et al. Impact of vitamin D on chronic kidney diseases in non-dialysis patients: A meta-analysis of randomized controlled trials. PLoS One. 2013;8 (4): 1–14. [ Links ]
66. Li XH, Feng L, Yang ZH, Liao YH. Effect of active Vitamin D on cardiovascular outcomes in predialysis chronic kidney diseases: A systematic review and meta-analysis. Nephrology. 2015; 20 (10): 706–14. [ Links ]
67. Kandula P, Dobre M, Schold JD, Schreiber MJ, Mehrotra R, Navaneethan SD. Vitamin D supplementation in chronic kidney disease: A systematic review and meta-analysis of observational studies and randomized controlled trials. Clin J Am Soc Nephrol. 2011; 6 (1): 50–62. [ Links ]
68. Shroff R, Wan M, Nagler E V., Bakkaloğlu S, Fischer D-C, Bishop N, et al. Clinical practice recommendations for native vitamin D therapy in children with chronic kidney disease Stages 2–5 and on dialysis. Nephrol Dial Transplant. 2017; 32 (7): 1098–113. [ Links ]
69. Shroff R, Wan M, Nagler E V., Bakkaloǧlu S, Cozzolino M, Bacchetta J, et al. Clinical practice recommendations for treatment with active Vitamin D analogues in children with chronic kidney disease Stages 2-5 and on dialysis. Nephrol Dial Transplant. 2017; 32 (7): 1114–27. [ Links ]
70. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011; 96 (7): 1911–30. [ Links ]
71. Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, et al. Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005; 90 (6): 3215–24. [ Links ]
72. Holick MF. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. 2017; 18 (2): 153–65. [ Links ]
73. Holick Michael, Biancuzzo Rachael CT et al. vitD2 igual a vitD3 Holick 2008. J Clin Endocrinol Metab. 2008; 93 (3): 677–81. [ Links ]
74. Biancuzzo RM, Clarke N, Reitz RE, Travison TG, Holick MF. Serum concentrations of 1,25-dihydroxyvitamin D2 and 1,25-dihydroxyvitamin D3 in response to vitamin D2 and vitamin D3 supplementation. J Clin Endocrinol Metab. 2013; 98 (3): 973–9. [ Links ]
75. Matias P, Jorge C, Ferreira C, Borges M, Aires I, Amaral T, Gil C, Cortez J FA. Cholecalciferol supplementation in hemodialysis patients: Effects on mineral metabolism, inflammation, and cardiac dimension parameters. Clin J Am Soc Nephrol. 2010; 5 (5): 905–11. [ Links ]
Cristina Jorge, MD
Nephrology Department
Hospital de Santa Cruz – Centro Hospitalar de Lisboa Ocidental
Av. Prof. Dr. Reinaldo dos Santos, 2790-134 Carnaxide
E-mail: cristinamjorge@gmail.com
Disclosure of potential conflicts of interest: none declared
Received for publication: Aug 10, 2019 Accepted in revised form: Aug 24, 2019














