Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Portuguese Journal of Nephrology & Hypertension
Print version ISSN 0872-0169
Port J Nephrol Hypert vol.33 no.1 Lisboa Mar. 2019
https://doi.org/10.32932/pjnh.2019.04.013
NEPHROPATHOLOGY QUIZ
Unmasked by histology’
Filipa Cardoso1, Joana Marques1, Bernardo Costa1, Mário Góis1,2, Helena Sousa1,2, Fernando Nolasco1
1 Nephrology Department, Hospital de Curry Cabral, Centro Hospitalar de Lisboa Central
2 Laboratory of Renal Morphology, Nephrology Department, Hospital Curry Cabral – Centro Hospitalar de Lisboa Central
CLINICAL PRESENTATION
We present the case of a 78-year-old male, with a known history of benign prostatic hyperplasia, hemorrhoids and paroxysmal atrial fibrillation (AF), with no history of kidney dysfunction. He presented to the emergency room three days after an elective sclerotherapy procedure for hemorrhoids with diarrhea, exhaustion and lack of appetite. He also mentioned having a fever (40ºC) on the day of the procedure that resolved with paracetamol. His vital signs were normal. Blood tests showed hemoglobin (Hb) 15.1 g/dL; acute kidney injury (creatinine 4.56mg/dL, urea 108mg/dL], elevated inflammatory markers [leukocytes 14x10^9/L, neutrophils 13x10^9/L, c-reactive protein (CRP) 372.9mg/L] and increased blood lactates 7.9 mmo/L. Urinalysis showed a specific gravity of 1.010, occasional proteinuria of 100 mg/dL, glycosuria of 50mg/dL and erythrocyturia of 823 u/L (on urinary catheterization). Kidney ultrasound showed normal-sized kidneys with normal corticomedullary differentiation.
He underwent an abdominal computed tomography (CT) with intravenous contrast that showed a prostatic abscess with a posterior fistula to the rectum. Shortly after his admission, and even though he started empirical therapy with piperacillin/tazobactam, he went into septic shock that motivated admission to an intensive care unit (ICU). Despite intense fluid resuscitation, he remained hypotensivewith need for vasopressor drugs for the first 24 hours. Later, the patient remained oligoanuric and initiated hemodialysis. He completed 14 days of antibiotic therapy for an Escherichia coli identified in blood cultures. Even though low urine output persisted, he made a progressive clinical recovery and was transferred to the nephrology ward. Elevated inflammatory parameters persisted (leukocytes 14.67x10^9/L, neutrophils 12.82x10^9/L, CRP 292 mg/L) and antibiotic therapy with ciprofloxacin was initiated, aiming to resolve a prostate infection since the prostatic abscess was still present in CT. He remained stable for about two weeks after which repeated episodes of AF with rapid ventricular response took place, reverted with amiodarone. Bilateral consolidation, compatible with pneumonia, was identified in a chest radiograph. This prompted an escalation of antimicrobial therapy, with little response. He became increasingly hypotensive, hypoxemic and dependent on high flow oxygen. Chest radiography was compatible with acute respiratory distress syndrome and he was admitted once again to an ICU. The patient developed respiratory failure and was intubated, remaining under invasive mechanical ventilation for 24 hours, with a favorable response. Vancomycin and meropenem were started with decrease of inflammatory parameters and four days later he was stable enough to be transferred to the ward. He completed 14 days of antimicrobial therapy, with steady improvement, despite no bacteria being identified in blood cultures. Blood work showed normocytic normochromic anemia, dependent on an erythropoiesis stimulating agent. Serum protein electrophoresis (SPE) showed alpha one and two spikes and a broad diffuse band in gamma region, compatible with an infectious disease process. Complete ionogram was normal. Despite the fact that he was now stable, he remained dialysis dependent and a kidney biopsy was performed.
QUESTIONS
1. What is the most likely diagnosis, considering the clinical history and presentation?
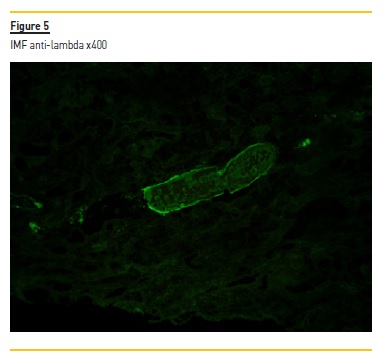
2. Considering light microscopy (Figures 1, 2, 3) and immunofluorescence studies (Figures 4, 5), what is the diagnosis?
3. What is our patient’s prognosis?
4. What are the treatment options?
ANSWERS
1. What is the most likely diagnosis, considering the clinical history and presentation?
Considering the prior clinical history, the renal image (both innocent), the absence of analytical signs of chronic renal disease (no anemia, hyperphosphatemia or hypocalcemia), as well as the successive occurrence of hemodynamic instability (septic shock, vasopressors), infectious and toxic insults (antibiotherapy, contrast), our main suspicion was acute tubular necrosis. However, the absence of improvement after stopping the abovementioned insults led us to perform a renal biopsy.
2. Considering light microscopy (Figures 1, 2, 3) and immunofluorescence studies (Figures 4, 5), what is the diagnosis?
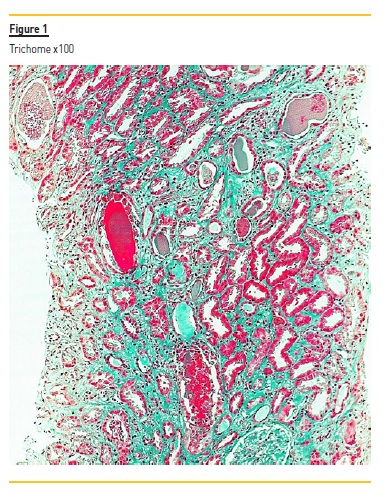
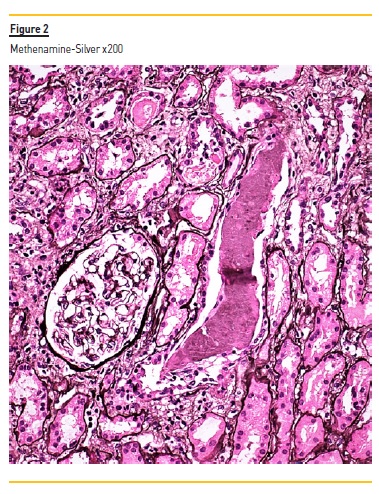
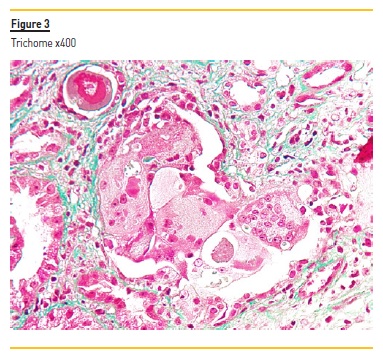
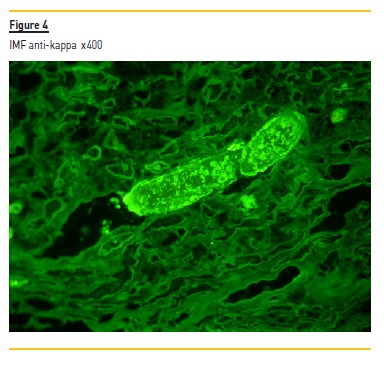
The biopsy specimen shows renal cortex with moderate interstitial fibrosis. The glomeruli were unremarkable. Figure 1 demonstrates several tubular casts (mainly in distal tubules), sharped-edged, that stained red in trichrome. These tubular casts also appeared fractured and lined by reactive tubular epithelial cells (Figure 2). Figure 3 shows giant cell reaction to intratubular casts. Tubular casts were strongly positive for kappa light chain (Figure 4), and the stain for lambda light chain was much weaker (Figure 5).
So, the histological result surprised us with the diagnosis of cast nephropathy. Consequently, we performed a complete workup to confirm it. A new M spike (myeloma gamma globulin) was identified on the SPE.
Also, serum free kappa light chains were elevated (KFLC = 50600mg/L) (rv 3.3-19.4), with normal free lambda light chains (LFLC = 21.5) (rv5.71-26.3) with a ratio of 2353.49 (rv 0.37-3.1). A myelogram was performed that showed 44% of plasmocytes; the bone biopsy had 50% plasmocytes.
The diagnosis of free kappa light chains multiple myeloma was made. Full skeletal radiography showed no lytic lesions. Beta-2 Microglobulin (B2M) 46.97mg/L was also elevated (vr 0.97-2.64), FISH results were negative for t(4;14) and t(14;16) translocations and p53 deletion.
3. What is our patient’s prognosis?
Until recently, approximately 25% of all newly diagnosed patients lived less than 3 years1. The introduction of new drugs (bortezomib, carfilzomib, panobinostat, daratumumab, elotuzumab, and ixazomib) has improved overall survival (OS) significantly. However, MM typically affects elderly patients, who face the challenge that treatment endurance is poorer and prognosis more unfavorable2. Furthermore, the simultaneous presence of additional diseases may complicate MM treatment5. For patients with newly diagnosed MM, correct and prompt staging of the disease is crucial to select the correct treatment approach and evaluate prognostic factors3.
To meet this aim, several staging systems are used, such as the Revised International Staging System (R-ISS), the Durie-Salmon (D&S) and the revised Myeloma Comorbidity Index (R-MCI)4-6. The first two showed great capability in differentiating patients with a worse prognosis from patients with a better outcome. Our patient had D&S IIIB and ISS 3, the worst possible categories, which are associated with the lowest overall survival rate. With growing numbers of elderly multiple myeloma patients, reliable tools to assess their vulnerability are required, that can be evaluated by R-MCI. Our patient fits in the second category, intermediate-fit (score 4–6). Additionally, renal impairment is a well-known risk factor for shorter survival in patients with MM. It is associated with a higher rate of treatment-related toxicity, early mortality and reduced OS, especially when dialysis support is required. As such, the prognosis for patients with MM is highly related to reversibility of renal failure7.
Of note, our patient was negative for high-risk cytogenetic abnormalities [t(4;14) and t(14;16) translocations and p53 deletion], but still belongs to R-ISS stage III due to high B2M. Cytogenetic assessment is essential for clinical practice, and the importance of this evaluation is indicated by its recent incorporation into the R-ISS for MM as an independent predictor of poor outcome in patients requiring dialysis and independently of renal function recovery8.
In conclusion, our patient has an unfavorable prognosis, not only because he has a high tumor burden, but also advanced age and he is on dialysis. Moreover, the multiple confusing causes of acute renal lesion and the atypical presentation contributed to a delayed diagnosis which worsens the overall prognosis. Therefore, not only did we lose precious time to potentially save the kidney, but he also was exposed to several factors that further damaged kidney function, which makes it even more unlikely that the patient will recover.
4. What are the treatment options?
Treatment should be initiated in all patients with MM. For patients in good clinical condition (e.g. fit patients) or those under 65 years old, induction followed by high-dose therapy with autologous stem cell transplantation is the standard treatment. For those who are not eligible, bortezomib / melphalan / prednisone (VMP) or lenalidomide plus low-dose dexamethasone (Rd) are the first -ine therapies. Because lenalidomide is associated with renal insufficiency, our patient was started on VMP regimen. This consists of bortezomib (1.3 mg/m2 subcutaneously days 1, 8, 15, 22), melphalan (9 mg/m2 orally days 1–4) and prednisone (60 mg/m2 orally days 1–4), repeated every 35 days.
Regarding the use of extracorporeal removal of FLCs with high cut-off hemodialysis using a dialysis membrane with a higher molecular weight cut-off pore size showed no benefit compared to conventional high-flux dialyzers as long as the patient is on a bortezomib-based triple regimen and is actually not recommended in cast nephropathy9,10.
References
1. Landgren O, Rajkumar SV. New developments in diagnosis, prognosis, and assessment of response in multiple myeloma. Clin Cancer Res. 2016 Nov 15;22(22):5428-5433. [ Links ]
2. Engelhardt M, Domm AS, Dold SM et al. A concise revised Myeloma Comorbidity Index as a valid prognostic instrument in a large cohort of 801 multiple myeloma patients. Haematologica. 2017 May;102(5):910-921. [ Links ]
3. Filonzi G, Mancuso K, Zamagni E et al. A comparison of different staging systems for multiple myeloma: can the MRI pattern play a prognostic role?. Am J Roentgenol. 2017 Jul;209(1):152-158. [ Links ]
4. Rajkumar SV, Fonseca R, San Miguel JF. Diagnosis and staging of multiple myeloma and related disorders. In Multiple Myeloma and Other Plasma Cell Neoplasms 2018 (pp. 17-28). Springer, Cham. [ Links ]
5. Rajkumar SV. Updated diagnostic criteria and staging system for multiple myeloma. American Society of Clinical Oncology Educational Book. 2016 May;36:e418-423. [ Links ]
6. Engelhardt M, Terpos E, Kleber M, et al. European Myeloma Network recommendations on the evaluation and treatment of newly diagnosed patients with multiple myeloma. Haematologica. 2014;99(2): 232-242. [ Links ]
7. Knudsen LM, Hjorth M, Hippe E, Nordic Myeloma Study Group. Renal failure in multiple myeloma: reversibility and impact on the prognosis. European journal of haematology. 2000 Sep;65(3):175-181. [ Links ]
8. Rajan AM, Rajkumar SV. Interpretation of cytogenetic results in multiple myeloma for clinical practice. Blood cancer journal. 2015 Oct;5(10):e365. [ Links ]
9. Cook M, Hutchison C, Fifer L, et al. High cut-off haemodialysis (HCO-HD) does not improve outcomes in myeloma cast nephropathy: results of European trial of free light chain removal by extended haemodialysis in cast nephropathy (EULITE). 21st Congress of the European-Hematology-Association. [ Links ]
10. Bridoux F, Carron P, Pegourie B et al. Effect of High-Cutoff Hemodialysis vs Conventional Hemodialysis on Hemodialysis Independence Among Patients With Myeloma Cast Nephropathy. JAMA. 2017;318(21):2099. [ Links ]
Mário Góis, MD
Laboratory of Renal Morphology Hospital Curry Cabral Centro Hospitalar de
Lisboa Central, Lisboa, Portugal
E-mail: mario.gois@chlc.min-saude.pt
Disclosure of potential conflicts of interest: none declared.
Authors’ Contributions: equal autorship of Filipa Cardoso and Joana Marques.
Received for publication: Mar 20, 2019
Accepted in revised form: Mar 22, 2019














