Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.32 no.4 Lisboa dez. 2018
TUBULAR QUIZ
Hyperkalemia: this time, not the usual suspect
Anna Lima, Pedro Campos, Ana Gaspar, Afonso Santos, Patricia Carrilho
Department of Nephrology, Hospital Prof Dr Fernando Fonseca, Lisbon, Portugal
A 49-year-old Caucasian male was admitted to the emergency department with a three-day history of abdominal pain and nausea. These episodes were frequent and, in the previous four months, he had noticed fatigue, more severe in the lower limbs, and symptoms compatible with postural hypotension. He had no fever or any other symptoms. Lab tests requested by his general practitioner six weeks earlier had revealed hyperkalemia, without other relevant changes. He had followed a low-potassium diet since then. Past medical history included active smoking habits, intravenous drug use (he suspended seventeen years ago), and changed bowel habits with alternating obstipation and diarrhea. He did not take any medication on a regular basis. Physical examination on admission revealed hyperpigmentation of the skin (more pronounced in the face) and hypotension (94/62mmHg). Laboratory testing revealed hyperkalemia (K= 6.48mmol/L), hyponatremia (Na=125mml/L), hypochloremia (Cl=89.8mmol/L). Serum creatinine (sCr) was 0.92mg/dl, urea 82mg/dl, and glycemia (non-fasting) 157mg/dl.
Arterial blood gas (FiO2=21%) showed metabolic acidosis (pH 7.38; pCO2= 23.6mmHg; pO2=121mmHg; HCO3=14 mEq/L; Na+=123 mmol/L; K+=5.26 mmol/L; Cl-=97 mmol/L; lactate 0.96mmol/L). Spot urine revealed pH=5, with no other abnormalities. Urinary sodium concentration was 123mmol/L, potassium 65mmol/L, and chloride 114mmol/L. Chest plain X-ray and electrocardiogram were normal.
WHAT IT THE MOST LIKELY CAUSE FOR HYPERKALEMIA AND METABOLIC ACIDOSIS IN THIS PATIENT?
This patient presented with nonspecific symptoms, associated with electrolyte and acid-base abnormalities in the laboratory results. In the presence of metabolic acidosis, one important step to search for the cause is to calculate the anion gap.1 In this case the anion gap was normal (AG= Na – (CL+HCO3) =12mmol/L). The next step was to calculate the urinary anion gap (UAG= (Na++ K+) – Cl-= 74mmol/L), which was highly positive. So, we were in presence of a metabolic acidosis with nonincreased anion gap, positive UAG, hyperkalemia and acidic urine (pH 5) in a patient with normal renal function matching a type IV (distal) renal tubular acidosis.2
There are many causes for hyperkalemic distal renal tubular acidosis, the most common of which is diabetes mellitus (by definition a hyporeninemic hypoaldosteronism).
Other important causes include drugs (for example those that interfere with the renin-angiotensin-aldosterone axis, nonsteroidal anti-inflammatory agents, heparin or ketoconazole), and states associated with mineralocorticoid deficiency (such as adrenal insufficiency and congenital enzyme defects).2,3 The patient had no history of diabetes and fasting glucose was within normal values (81mg/dL). He denied recent use of any drugs prescribed, over-the-counter, or recreational.
Given the exclusion of the most frequent causes of distal renal tubular acidosis, the most likely cause for the symptoms and laboratory changes is adrenal insufficiency. This disease usually presents with insidious signs and symptoms derived from the glucocorticoid and mineralocorticoid deficiency: fatigue, weight loss, salt craving, gastrointestinal complaints, skin hyperpigmentation and postural hypotension.4,5
Accompanying laboratory findings include hyponatremia, hyperkalemia and anemia. This patient had most of the described features. Patients with chronic adrenal insufficiency may present with glucocorticoid or mineralocorticoid deficiency. Diagnosis is difficult since the onset is insidious and the associated symptoms are nonspecific. Since adrenal insufficiency was suspected, we evaluated the renin-angiotensin-aldosterone axis and the pituitary-adrenal axis [Table 1].2
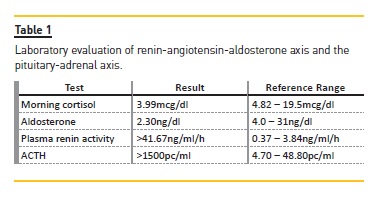
Laboratory tests revealed a low morning serum cortisol concentration (3.99mcg/dl) with very high corticotropin (ACTH >1500pg/ml) and low aldosterone (2.3 ng/dl) in the presence of elevated plasma renin activity (>41.67ng/ml/h) compatible with primary adrenal insufficiency. In order to confirm the diagnosis, an ACTH stimulation test was performed (Synacthen test) [Table 2].
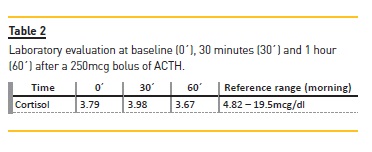
A normal response to this test is a rise in cortisol serum concentration after the infusion of ACTH to a peak >18-20mcg/dl, which was not present in this patient, thus confirming the diagnosis of adrenal insufficiency. After the diagnosis, a treatable cause was pursued.4,5 The patient was tested for HIV (important in view of his previous intravenous drug use) which was negative; there was no previous history of tuberculosis or autoimmune disease, and abdominal CT-scan revealed adrenal glands with relatively small dimension with the left gland demonstrating discrete structural heterogeneity.Antibody against 21-alpha-hidroxilase was negative.
Thyroid and parathyroid function were normal (TSH 1.2mU/L; Free T4 0.9ng/dL; PTH 50ng/L). He was started on hydrocortisone and fludrocortisone with resolution of the symptoms described and normalization of the lab tests.
WHY DOES THIS PATIENT HAVE HYPERKALEMIA AND METABOLIC ACIDOSIS?
Aldosterone is produced in the zona glomerulosa of the adrenal cortex and is responsible for the electrolyte management in the principal and intercalated cells of the distal renal tubules (sodium, potassium, and hydrogen). The main goal of aldosterone secretion is to increase extracellular volume.2
Aldosterone binds to its receptor in the tubular cells and promotes the expression of various tubular enzymes (pumps and channels) that promote sodium reabsorption and potassium excretion. The most important are the Na+/K+ ATPase pump, located in the basolateral membrane, and the epithelial sodium channel (ENaC) located in the luminal membrane. The Na+/K+ ATPase pump sets up a lumen-negative transepithelial potential difference which promotes the drive for sodium entrance in the ENaC. Thus, patients with deficient aldosterone production often have clinically significant hyperkalemia due to accumulation of potassium in the extracellular space and renal retention of potassium.6 Additionally, aldosterone deficiency promotes sodium loss in the urine and hence volume depletion, which is the main cause of postural hypotension. This often leads to a diminished serum sodium concentration because of activation of anti-diuretic hormone (ADH) which promotes free-water reabsorption from the distal tubule and dilutes the plasma sodium.2,3 In this patient, the glucocorticoid deficiency also had an effect on the electrolyte disturbances. Glucocorticoid deficiency contributes to hyponatremia by several mechanisms: altered water permeability in the collecting tubules in an ADHindependent manner and decreased glomerular filtration rate secondary to hypotension caused by glucocorticoid deficiency.7
In the acid-base area, aldosterone promotes secretion of hydrogen ions in exchange for reabsorbed potassium in the intercalated cells of the cortical and collecting tubules. Its absence increases the hydrogen ion (H+) concentration in the extracellular fluid, causing metabolic acidosis. In addition, distal acidification of the urine is impaired, due to diminished sodium reabsorption in the principal cells, promoting a decrease in lumen electronegativity and consequently decreased driving force for H+ secretion. Alongside changes in potassium also influence hydrogen excretion. Hyperkalemia interferes with the ammonia metabolism (NH3/NH4+): it suppresses ammonia synthesis in the proximal tubule, resulting in low urinary NH4+.2,3 These changes lead to a reduced availability of ammonia as a urinary buffer, reduced H+ secretion and development of metabolic acidosis.
The case presented depicts the tubular changes associated with hormonal dysregulation of the adrenal axis and shows the importance of being aware of the not so frequent causes of electrolyte and acid-base disorders.
References
1. Kraut JA, Madias NE. Serum anion gap: its uses and limitations in clinical medicine. Clin J Am Soc Nephrol. 2007;2(1):162-74. [ Links ]
2. John F, Jurgen F, Marcello T, Richard J. Comprehensive Clinical Nephrology. Sixth Edition ed2019. [ Links ]
3. HALL J. Guyton & Hall Textbook of Medical Physiology2015. [ Links ]
4. Bancos I, Hahner S, Tomlinson J, Arlt W. Diagnosis and management of adrenal insufficiency. Lancet Diabetes Endocrinol. 2015;3(3):216-26. [ Links ]
5. Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, Hammer GD, et al. Diagnosis and Treatment of Primary Adrenal Insufficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101(2):364-89. [ Links ]
6. Lee Hamm L, Hering-Smith KS, Nakhoul NL. Acid-base and potassium homeostasis. Semin Nephrol. 2013;33(3):257-64. [ Links ]
7. Palmer BF, Glassock RJ, Bleyer AJ. American Society of Nephrology Quiz and Questionnaire 2012: Electrolytes. Clin J Am Soc Nephrol. 2013;8(6):1048-53. [ Links ]
Anna Lima MD
Department of Nephrology, Hospital Prof Dr Fernando Fonseca
Email: anna.lima@hff.min-saude.pt
Disclosure of potential conflicts of interest: None declared
Received for publication: Dec 18, 2018
Accepted in revised form: Jan 4, 2019














