Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.32 no.3 Lisboa set. 2018
NEPHROPATHOLOGY QUIS
What seems most likely may not be the case
Filipa Cardoso1, Joana Marques1, Marco Mendes1, Mário Góis1,2, Helena Sousa1,2, Fernando Nolasco1
1 Nephrology Department, Hospital de Curry Cabral, Centro Hospitalar de Lisboa Central
2 Laboratory of Renal Morphology, Nephrology Department, Hospital Curry Cabral - Centro Hospitalar de Lisboa Central
CLINICAL PRESENTATION
A 78-year-old Caucasian woman with a past medical history of hypertension, type 2 diabetes mellitus and dyslipidemia presented to the emergency department with fever (38°C), arterial hypertension (223/98mmHg) and non-bloody diarrhea. She also referred progressive weakness and worsening of her general condition in past weeks. Laboratory testing showed anemia (10.6 g/dL) and elevated inflammatory parameters (12840 leukocytes and a c-reactive protein of 102.9 mg/L).
Kidney function was normal. She was admitted to a medical ward with the diagnosis of gastroenteritis and treated with a 7-day course of ciprofloxacin with clinical improvement. The blood and stool cultures were negative.
For further investigation of anemia and clinical findings, a complete workup was started that showed elevated IgM (8.5 g/L, decreased IgG (1.94 g/L), a monoclonal component IgM kappa, free kappa light chains of 422 mg/L, free lambda light chains of 22.9 mg/L with a ratio of 18.43. A full-body computed tomographyshowed no lytic lesions and no organ or adenomegaly. A myelogram was performed that showed 7% plasmocytes. Bone biopsy had a 5% infiltration of CD138+ plasmocytes.
After a few days, the patient complained of intense pain in her both hands with decreased sensibility and mobility. Simultaneously, kidney function started to deteriorate with a serum creatinine of 1.66 mg/dL. The urinalysis showed microhematuria and proteinuria.
Based upon these results, the patient was transferred to a nephrology ward where we performed a more detailed blood tests that showed a serum albumin of 30.7 g/L, proteinuria of 4.3 g/24h, normal C3 with a decreased C4 (0.04 g/dL), an elevated rheumatoid factor of 17800 UI/mL and a type II mixed cryoglobulinemia with monoclonal IgM. Beta-2 microglobulin (B2M) was elevated 27.34mg/L. Immunological panel including ANA, ANCA, anti-GBM and serologic testing including HIV, hepatitis C and B virus was negative. A renal biopsy was performed.
QUESTIONS
1. According to the clinical, blood and histological findings, what is the most likely diagnosis?
2. What treatment options do we have in this case? Is plasma exchange an option?
3. In this patient, what are the renal and vital prognosis?
ANSWERS
1. According to the clinical, blood and histological findings, what is the most likely diagnosis?
Figure 1 represents two glomeruli with mesangial proliferation and endocapillary hypercellularity due to mononuclear cells, with double contours of glomerular basement membranes. The image also shows a pseudothrombi (hyaline thrombi) in afferent arteriole.
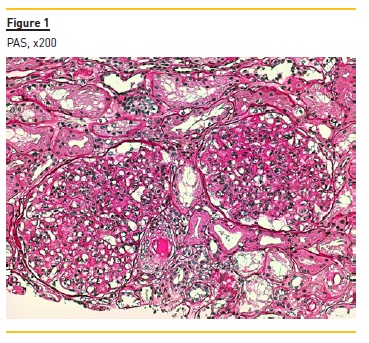
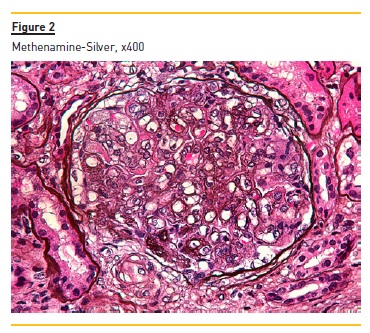
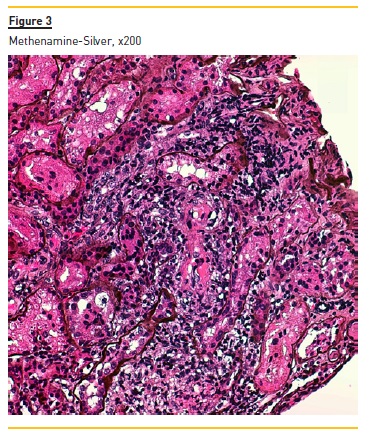
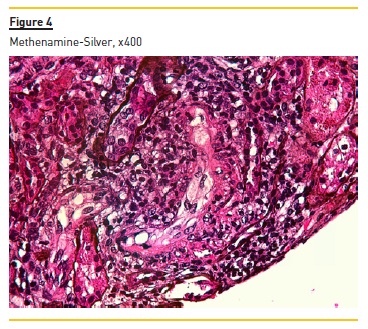
Figure 2 shows widespread duplication of glomerular basement membrane, endotheliosis and subendothelial hyaline deposits. A cryoglobulinemic vasculitis (with cryoglobulin deposits, arteriolar inflammation and endotheliosis) is shown in figure 3 and 4.
Immunofluorescence (paraffin-embedded tissue sections) showed subendothelial and endocapillary deposits of IgM (+++) (figure 5) and kappa (+++) (Figure 6). Lambda was negative (not shown).
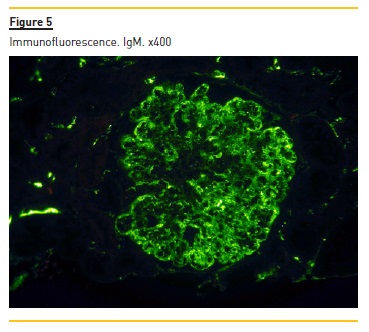
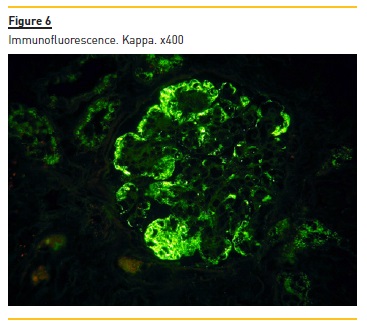
Our patient presented with anemia, renal failure and peripheral neuropathy. The laboratory workup showed a monoclonal component IgM kappa, elevated IgM, increased free kappa light chains with a ratio of 18.43 and bone marrow infiltration by plasmocytes CD138+.
Waldenström macroglobulinemia (WM) is a rare lymphoproliferative disorder characterized by the proliferation of lymphoplasmacytic elements in the bone marrow and the presence of IgM gammopathy. It can be distinguished clinically from IgM myeloma by the lymphoplasmacytic versus pure plasmacytic morphology, absent versus present bone involvement, and immunophenotypic findings1-3. Considering all of the above, our patient was diagnosed with WM.
Membranoproliferative glomerulonephritis (MPGN) is a glomerular-injury pattern common to an heterogenous group of diseases, corresponding to 7-10% of all GN biopsyproven.
Based on histologic diagnosis, it is divided into two groups which can be distinguishable by immunofluorescente: MGPN mediated by immunocomplexes or by complement.
The first results from persistent antigenemia or from circulating immunocomplexes (due to autoimmune diseases or paraproteinemia); our patients fits into immunocomplex-mediated MPGN secondary to MW4.
Furthermore, the blood tests identified a type II mixed cryoglobulinemia with rheumatoid factor activity.
Cryoglobulins in mixed cryoglobulinemia result from a B-cell lymphoproliferative process in the setting of persistent immune activation triggered by chronic infection, autoimmune disease, or an unknown cause. In our case, they are secondary to WM5-7. Considering clinical, histological and blood findings, our final diagnosis is a type II mixed cryoglobulinemia secondary to WM with an MPGN pattern on kidney biopsy.
2. What treatment options do we have in this case? Is plasma exchange an option?
Given the rarity of non-HCV mixed cryoglobulinemia, it is challenging to provide evidence-based treatment recommendations. The type of treatment for cryoglobulinemic vasculitis depends on its underlying cause.
Treatment choice should take into account patients age and characteristics, specific goals of therapy, necessity for rapid disease control, risk of treatment-related neuropathy, immunosuppression, secondary malignancies and potential for future autologous stem cell transplantation (ASCT). High-dose chemotherapy paired with ASCT is the standard frontline treatment for younger and fit patients. However, some are ineligible due to age, comorbidities, impaired fitness or disability. Our patient fits into this category.
Patients with severe or life-threatening manifestations (such as kidney impairment) need urgent intervention to suppress immune complex formation. This is accomplished with immunosuppressive therapy (IST), which is primarily based on high-dose corticosteroids, cyclophosphamide, rituximab and plasmapheresis. She started treatment with DRC protocol (Dexamethasone 20 mg IV day 1, Rituximab 375 mg/m2 IV day 1, Cyclophosphamide 100 mg/m2 PO BID days 1-5, repeat 21 day cycle for 6 cycles) (8-10).
Because IgM is a pentamer with molecular size of 925 kDa, it can exert profound effects on blood cells and blood flow, especially when present in high concentrations.
Whereas serum viscosity rises linearly with increasing IgG levels, the increase in viscosity with rising IgM concentration can become exponential above a concentration of 30 g/L. This can lead to high viscosity syndrome (HVS). Patients with HVS have skin and mucosal bleeding, retinopathy with visual disturbances, and a variety of neurologic symptoms varying from headache to coma.
It can be diagnosed from the physical examination by identifying characteristic retinal venous engorgement (sausaging) on funduscopic inspection11. Even though our center does not measure viscosity and therefore we could not safely refute HVS, our patient had a IgM level of 8.5 g/L; no HVS symptoms apart from headaches which could be attributed to her high blood pressure, and her fundoscopy was normal. As such, we believe that plasmapheresis was not indicated.
3. In this patient, what are the renal and vital prognosis?
Despite significant advances in our understanding of WM, it remains incurable. The goals of management of this typically indolent malignancy are to mitigate disease-related symptoms and decrease the risk of organ damage without incurring significant toxicity, particularly because a high proportion of the WM patients ultimately receive more than a single line of therapy during the course of their disease12.
The international prognostic scoring system for WM takes five adverse features into account (age of more than 65 years, hemoglobin less than or equal to 11.5g/dl, platelet count of less than or equal to 100 × 109/l, B2M more than 3 mg/l and serum IgM concentration more than 70 g/l). Our patient has 3 which puts her at intermediate / high risk, with a 5-year survival rates of 36%13. The presence of cryoglobulins per se does not worsen the mortality or morbidity risk6.
All things considered, our patients vital and renal prognosis are poor. Even though her renal prognosis is closely linked to her hematological response, her kidney damage is worse than one would expect given the tumor burden she had at the time of the biopsy, hinting at an even inferior prognosis.
References
1. Kastritis E, Leblond V, Dimopoulos MA et al, Waldenstroms macroglobulinaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018; (Suppl 0): iv1–iv10. [ Links ]
2. Owen R, Treon SP, Al-Katib A et al, Clinicopathological Definition of Waldenstroms Macroglobulinemia: Consensus Panel Recommendations From the Second International Workshop on Waldenstroms Macroglobulinemia, Semin Oncol. 2003 Apr;30(2):110-5 [ Links ]
3. Higgins L, Nasr SH, Said SM et al, Kidney Involvement of Patients with Waldenstrom Macroglobulinemia and Other IgM-Producing B Cell Lymphoproliferative Disorders. Clin J Am Soc Nephrol. 2018 Jul 6;13(7):1037-1046. [ Links ]
4. Sethi S, Fervenza F. Membranoproliferative Glomerulonephritis - A New Look at an Old Entity. N Engl J Med 2012; 366:1119-1131. [ Links ]
5. Damoiseaux J, Cohen Tervaert JW. Diagnostics and Treatment of Cryoglobulinaemia: It Takes Two to Tango. Clin Rev Allergy Immunol. 2014 Dec;47(3):299-310. [ Links ]
6. Overview of cryoglobulins and cryoglobulinemia, UPTODATE, consulted on 27-08-2018. [ Links ]
7. Blanco P, Viallard J, Rivel J, et al. Unusual manifestations of type II cryoglobulinaemia associated with Waldenströms macroglobulinaemia, J Clin Pathol. 2000;53:882-884. [ Links ]
8. Paludo J. et al, Dexamethasone, rituximab and cyclophosphamide for relapsed and/or refractory and treatment-naıve patients with Waldenstrom macroglobulinemia, British Journal of Haematology, vol. 179, pg.: 98–105, August 2017 [ Links ]
9. Muchtar E, Magen H, Gertz MA. How I treat cryoglobulinemia. Blood. 2017 Jan 19;129(3):289-298 [ Links ]
10. Treon SP. How I treat Waldenstrom macroglobulinemia, Blood. 2015 Aug 6;126(6):721-32. [ Links ]
11. Stone MJ, Bogen SA. Role of Plasmapheresis in Waldenströms Macroglobulinemia. Clin Lymphoma Myeloma Leuk. 2013 Apr;13(2):238-40. [ Links ]
12. Treatment and prognosis of Waldenström macroglobulinemia, UPTODATE, last consulted on 25-09-2018. [ Links ]
13. Kastritis E, Kyrtsonis MC, Hadjiharissi E, et al. Validation of the International Prognostic Scoring System (IPSS) for Waldenstroms macroglobulinemia (WM) and the importance of serum lactate dehydrogenase (LDH). Leuk Res. 2010 Oct;34(10):1340-3. [ Links ]
Helena Sousa, MD
Laboratory of Renal Morphology Hospital Curry Cabral
Centro Hospitalar de Lisboa Central, Lisboa, Portugal
E-mail: iana.helena@gmail.com
Disclosure of potential conflicts of interest: none declared.
Received for publication: Sep 25, 2018
Accepted in revised form: Sep 26, 2018














