Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Portuguese Journal of Nephrology & Hypertension
Print version ISSN 0872-0169
Port J Nephrol Hypert vol.32 no.3 Lisboa Sept. 2018
ORIGINAL ARTICLE
Nephrolithiasis in a portuguese pediatric population
Joana V. Andrade1,2, Sofia Bota2, Telma Francisco2, Raquel Santos2, Gisela Neto2, Margarida Abranches2
1 Centro Hospitalar Tondela‑Viseu, EPE, Viseu, Portugal
2 Pediatric Nephrology Unit, Hospital de Dona Estefânia, Centro Hospitalar de Lisboa Central, Lisbon, Portugal
ABSTRACT
Introduction and Aims: Nephrolithiasis incidence in children has increased considerably. It is associated with substantial morbidity, recurrence and increased adulthood cardiovascular risk and chronic kidney disease. A thorough investigation is essential, as rare forms of urolithiasis have increased risk of renal failure. We aim to determine the epidemiology and outcomes of a pediatric population with nephrolithiasis presented in a nephrology unit of a tertiary centre.
Methods: Retrospective study of the records of all children (<18 years) with nephrolithiasis diagnosis between 2008‑17. Clinical features, etiology, recurrence, treatment, and outcomes were evaluated and compared throughout the study period through two equal periods (2008‑12 versus 2013‑17).
Results: We identified 80 cases: isolated nephrolithiasis (86%) and associated with nephrocalcinosis (14%). Mean follow‑up was 36 months (14–120). Median age at presentation was 8.6 years [3 months – 17 years]: 21% < 2 years‑old and 46% ≥ 10 years. The annual ratio of referrals for nephrolithiasis increased on average 1.2% per year [0.3‑11.8%]. Multiple etiological factors were present in 34%. A metabolic abnormality was identified in 54%: hypocitraturia (34%), hypercalcuria (24%), hyperoxaluria (15%), hyperuricosuria (15%) and cystinuria (1%), without age predominance (p=0.2). Urinary tract infection (24%) was the next most significant etiology and was more frequent below 2 years of age (p=0.001) and associated with struvite calculi (p=0.033). Median age at diagnosis was significantly lower in the studys first half (5 vs 10 years; p=0.019) and an infectious etiology was more frequent (p=0.043). In a logistic‑regression analysis, a family history of nephrolithiasis was associated with a metabolic cause (p<0.01). Sixty‑three percent became stone free and 24% had recurrence.
Discussion: Nephrolithiasis new referrals gradually increased throughout the study period. The most common etiology was metabolic, which is usually responsible for nephrolithiasis appearance and its recurrence, emphasizing the need for a complete evaluation.
Keywords: nephrolithiasis, kidney stone, children, Portuguese, metabolic disease, obesity
INTRODUCTION
Urolithiasis incidence in children has increased considerably over the last few decades and is associated with substantial morbidity and recurrence. An estimated prevalence of 1‑5% is reported in the United States of America and it is responsible for 1 in 1,000 pediatric hospital admissions, with a slight male predominance1‑4.
Recent epidemiological studies have suggested an increased incidence of kidney stone disease in all age groups, particularly in children4‑6.
The etiology of pediatric urolithiasis is generally undetermined and this rising incidence has been endorsed by many reasons, such as metabolic, anatomical, dietaryand environmental risk factors7‑12.
While in pediatric patients infection was considered the primary cause of urolithiasis13,14, the etiologic pattern in younger patients has changed from mainly infectious to metabolic causes13‑16.
Anatomical abnormalities, such as ureteropelvic junction obstruction or vesicoureteral reflux, are associated with infection and urinary stasis, thus enhancing stone formation17‑19.
Over the past decades, metabolic disorders have been recognized in 33–95% of pediatric patients. Several studies have demonstrated the implication of metabolic risk factors – such as hypercalciuria, cystinuria and hyperoxaluria – in children with urolithiasis etiology20‑24.
Influences of some environmental features have to be considered, such as family diet patterns and the higher risk of lithiasis among stone‑former relatives. Indeed, an increased consumption of salty fast food and high‑protein diets has been associated with an increase in urolithiasis.
On the other hand, it is well documented that hypercalciuria is significantly disturbed by dietary factors25: boosted by excessive calcium, sodium and animal protein intake and diminished by potassium and citrate (high in fruit and vegetables) ingestion26. It would appear that the eating of animal protein predisposes to the development of hyperuricemia, thereby creating an acid load, which increases the urinary excretion of calcium and uric acid and reduced citrate27. Chocolate contributes to an enlarged stone‑forming risk due to its oxalate content28. Ingesting a diet high in fruit and vegetables, moderate in low‑fat dairy products and low in salt and animal protein has been associated with reduced stone‑formation risk29,30. Low urinary volume may be the most important risk factor for stone formation in healthy individuals and no child with urinary flow rate higher than 1.6 ml/kg/h is at risk of stone formation31. Finally, an increase in stone disease has also been attributed to other factors, such as an increasingly sedentary lifestyle, the global rise in obesity, global warming and climate changes32. An association between increasing body mass index (BMI) and increasing urinary excretion of sodium, calcium, uric acid, and citrate has been reported33.
All children that present with urolithiasis warrant a thorough investigation because some rare forms of stone disease are associated with a different risk of renal failure34,35: adenine phosphoribosyltransferase deficiency, cystinuria, Dent disease, familial hypomagnesemia with hypercalciuria and nephrocalcinosis, and primary hyperoxaluria. Although rarely obtained, a key part of the pediatric kidney stone check‑up is the stone evaluation35. The urine chemistry is a crucial test, either from twenty‑four hour collections or random urine samples in younger children.
The aim of this study was to characterize the epidemiology, clinical features, metabolic risk factors, diagnosis, treatment and outcome of a pediatric population with stone disease evaluated at a tertiary centre nephrology unit. As secondary outcomes, we aim to determine recurrence risk factors and to assess if there were differences regarding epidemiology, etiology and clinical risk factors throughout the study period.
SUBJECTS AND METHODS
Study population
Retrospective review of medical records from all patients aged 0‑18 years referred to the Pediatric Nephrology
Ambulatory Department of a level III Hospital, from January 1st, 2008 to December 31st, 2017 with a nephrolithiasis diagnosis. Patients were divided into groups of equal period (2008‑12 and 2013‑17) and compared.
Clinical features, past medical conditions, renal lithiasis family history, etiology, treatment and outcomes were evaluated. Excessive dairy products, animal protein, oxalates, phytates and sodium were considered dietary errors, as was reduced water, fruit and vegetable intake. We defined obesity as a body mass index (BMI) superior to the 97th percentile and overweight between the 85th and 97th percentiles of BMI for age and gender of the World Health Organization charts.
Urolithiasis was always confirmed by ultrasonography. Voiding cystourethrography (VCUG) and isotopic renal scans (DMSA/MAG3 scan) were performed in selected cases: VCUG was performed in patients with recurrent urinary tract infection (UTI) and/or hydronephrosis; DMSA scan to identify renal scar and MAG3 scan if obstruction or hydronephrosis were suspected.
Workup
Blood serum with creatinine, urea nitrogen, uric acid, acid‑base status, sodium, potassium, chlorine, magnesium, phosphorus and urine analysis were performed at the first medical appointment. Urine cultures were conducted if there were clinical and laboratory findings suggestive of UTI. In patients with UTI, metabolic assessment was performed after the infection was treated. A stone risk panel was executed on 24‑hour urine samples. We considered hypercalcuria >4 mg/kg/day, hyperoxaluria >0.5 mmol (45 mg)/1.73m2/day, hyperuricosuria >815mg/1.73m2/day, cystinuria >75mg/1.73m2/day, hypocitraturia <320 mg/1.73m2/day, hypomagnesuria <2 mmol/day. Lithogenic risk was defined as urinary calcium/citrate ratio >0.3316,31,36,37.
Abnormal urinary excretion results were always confirmed in at least two samples.
Stone samples obtained by spontaneous passage, or open operation, were analysed by infrared spectroscopy analysis. The predominant component of the calculi was used to classify it.
Statistical analysis
The collected data was analysed by SPSS, 20th version software (SPSS, Chicago, IL) for Mac using Students T test, the Mann–Whitney U test, chi‑square test, Fishers exact test and logistic regression analysis, with P values of <0.05 considered to be significant.
Ethics
The procedures were in accordance with the ethical standards of the committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975 (revised in 2015).
RESULTS
We identified 80 cases in nine years of study: isolated nephrolithiasis (n=69, 86%) and associated with nephrocalcinosis (n=11, 14%). The annual ratio of referrals for nephrolithiasis increased on average 1.2% per year (2.3% requests in the first year – 11.8% the last year [Figure 1]). Mean follow‑up was 36 months (14–120).
Demographic characteristics
Median age at presentation was 8 years [minimum 3 months‑old – maximum 17 years‑old] with a (3‑14) interquartile range (Figure 1): 21% under 2 years old (n=17), 33% between 2‑10 years (n=26) and 46% with more than 10 years old (n=37). Male predominance was observed (n=47, 59%).
Regarding BMI, 4% (n=3) were under the 3rd percentile and 16% (n=13) above the 97th percentile (Table I). Figure 2 shows the BMI by year of study. We found no association between excessive weight and obesity (BMI > P85) and age above 10 years old (p=0.159).Figure 3
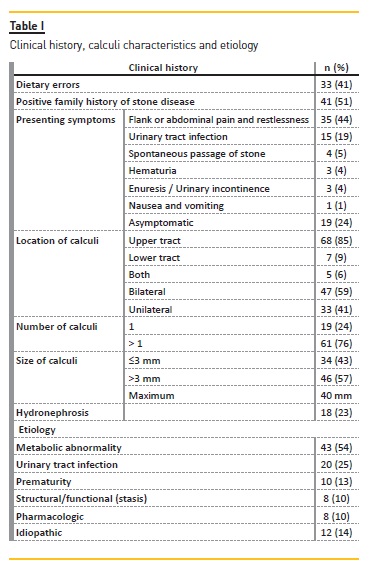
Presenting symptoms at diagnosis
The most common presenting symptom was flank or abdominal pain (44%, n=35), followed by UTI (19%, n=15) (Table I). Two patients had hypertension; one had chronic renal disease and the other an ureteropelvic junction syndrome. Four patients (5%) had spontaneous passage of stone (Table I).
In 24% (n=19) there were no symptoms and lithiasis was detected by ultrasound performed for syndromic diseases or structural malformations (11%, n=9), recurrent UTI evaluation (5%, n=4), prematurity follow‑up (4%, n=3), tubulopathy investigation (3%, n=2) and inflammatory bowel disease (1%, n=1).
The upper urinary tract was the most commonly affected site (85%, n=68) (Table I).
Etiology
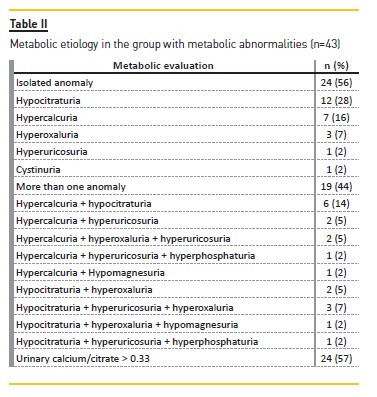
Multiple etiological factors were present in 34% (n=27) of patients. In 54% (n=43), urine evaluation revealed a metabolic disturbance (Tables II and III) and the majority (56%, n=24) had a single disturbance. Globally, the most common metabolic abnormalities were hypocitraturia (63%, n=27), followed by hypercalcuria (44%, n=19), hyperoxaluria (28%, n=12) and hyperuricosuria (28%, n=12). Lithogenic risk was present in 56% (n=24).
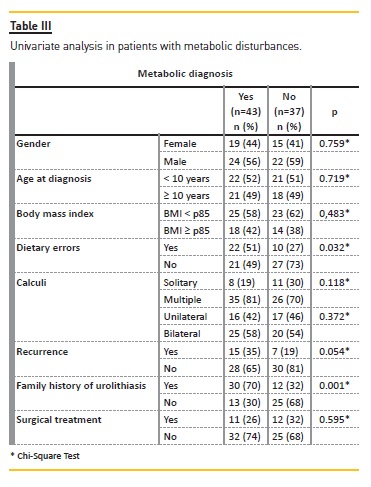
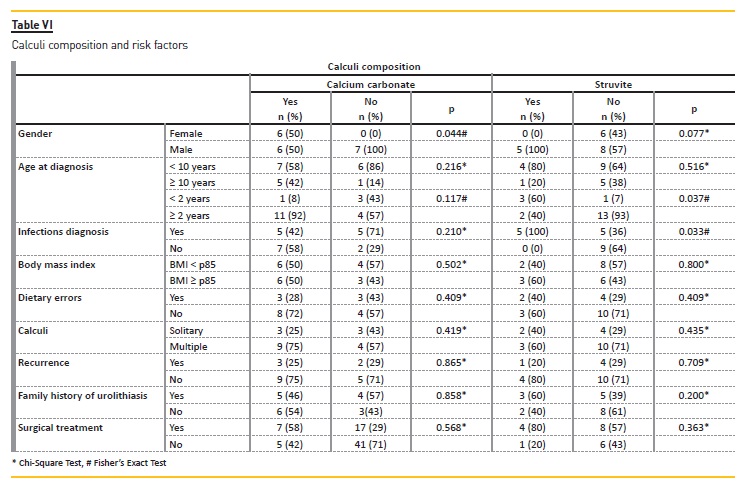
No gender (p=0.759) or age (p=0.719) with statistically significant predominance was observed (Table III). In a logistic‑regression analysis adjusted for age, sex, weight, dietary errors, type of calculi and recurrence of symptoms, a known family history of nephrolithiasis was associated with an increased risk for a metabolic cause (odds ratio = 6.86; 95% confidence interval, 1.663‑28.302; p<0.001).
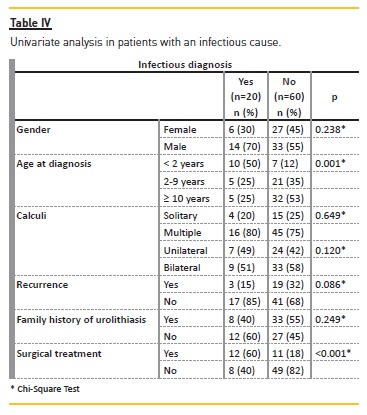
UTI was the second most significant etiology (25%, n=20) and functional/structural urinary anomalies were also an important cause (10%, n=8). Children below 2 years of age were more likely to have UTI as a cause (p=0.001), without gender predominance (p=0.238) (Table IV). Proteus mirabilis was the most frequent agent identified.
Other identified causes included prematurity and medications, such as vitamin D and corticosteroids (Table I).
In 14% (n=11) none of the previously mentioned causes were identified and therefore were considered idiopathic. Recognized possible risk factors for these cases were low urinary volume (55%, n=6), other dietary errors (55%, n=6) and family history of urolithiasis (36%, n=4). In the majority of these (63%, n=7), lithiasis fiureDietary errors and urinary output
Dietary errors were identified in 34% (n=27): excessive dairy products in 67% (n=18); reduced water intake in 48% (n=13); reduced fruit and vegetables in 33% (n=9); excessive phytate containing beverages in 19% (n=5), high intake of animal proteins in 15% (n=4); excessive salt 15% (n=4); excessive chocolate 7% (n=2).
Patients with dietary errors had a more frequent familial history of kidney stone (p=0.017) and a metabolic abnormality (p=0.032) (Table III).
Of the 68 patients evaluated for urinary output, we found that 37% (n=25) had low urine output (<1 ml/ kg/h).
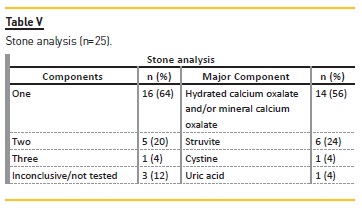
Stone analysis
Stone analysis (Tables V and VI) was available for 28% (n=22) (stone size 3‑40 mm), revealing a calcium carbonate oxalate and/or mineral calcium oxalate composition in more than half. The calcium carbonate composition was more frequently encountered in the female gender (p=0.044) and the struvite composition in children less than two years old (p=0.037). Struvite calculi were associated with infectious causes (p=0.033).
Evolution through the study period
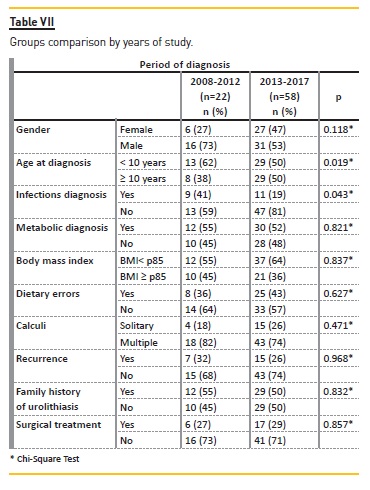
By dividing the sample in two groups – patients followed between 2008‑2012 and 2013‑2017 – we found that median age at diagnosis was significantly different (p=0.019): 5 years‑old in the first group and 10 years‑old in the latter, meaning adolescents became more frequently affected in recent years. Although we observed a gradual increase in overweight/obesity ratio throughout the study period, it was not more frequent in 2013‑2017 (p=0.653). In the 2008‑2012 group, an infectious etiology was more frequent (p=0.043) (Table VII).
Treatment
The majority was conservatively treated (71%, n=57). Surgical treatment was performed in 29% (n=23); extracorporeal shock‑wave lithotripsy (ESWL) in 14% (n=11) and, due to recurrence or failure of ESWL, 10% (n=8) underwent stent placement and 10% (n=8) open surgeries. The UTI cause was more likely to need surgical treatment (p<0.001) (Table IV).
Outcome
During follow‑up, 63% (n=50) of patients became stone free or diminished in size and 28% (n=22) had recurrence (Table VIII). No association was found between recurrence and metabolic (p=0.054) or infectious (p=0.087) causes or dietary errors (p=0.927).
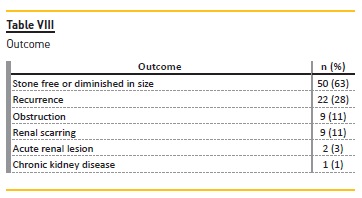
Complications were observed in 25% (n=20): acute renal lesion in 3% (n=2), obstruction in 11% (n=9), renal scarring in 11% (n=9) (Table VIII). One patient developed stage II chronic kidney disease (CKD) at the age of 13 years old, four years after the diagnosis of type 1 primary hyperoxaluria (AGXT homozygous mutation).
DISCUSSION
Incidence
Over the last decade an increasing incidence of kidney stone disease in children and adolescents has been reported, between 4 to 10% annually11,38‑40.
This study is not a population‑based study and our results might vary from the overall pediatric stone disease population in Portugal, but it may also reflect an increasing incidence in the general population, as we are one of five Portuguese tertiary pediatric centres.
In this study, a gradual increase of 1.2% per year (on average) of the proportion of referrals for nephrolithiasis was observed [minimum 0.3% in 2009 – maximum 11.8% in 2017]. The higher incidence may be explained by various reasons, including dietary errors12,15,27‑30, but we did not identify any significant cause in the studys second half group.
Age
Most pediatric series note a significant number of adolescents with kidney stones36. In our group, 46% were over 10 years. We ascertained a higher median age of presentation (10 vs 5 years‑old, p=0.019) and a higher adolescents proportion in the 2013‑2017 group.
Tasian et al.38,39 found a 52% higher annual incidence of nephrolithiasis among 15 to 19‑year‑old females than males the same age. Also, Dwyer MEs study40 had 36% of patients aged 12 to 17 years old and showed a threefold increased incidence in adolescents, while it remained stable in the younger age groups. However, the authors did not find a specific reason for this increase. In our population, we observed a decrease in infectious causes in the second half of the study (p=0.043), which was in relation with younger ages (<2 years‑old, p=0.001).
BMI and obesity
Also, although not statistically significant, we noticed an increase in the proportion of patients with excessive weight and obesity.
It has been hypothesized that the rising incidence of stone disease in pediatric populations is related to childhood obesity, but it still is not consensual, as shown by Dwyer et al.40 and, more recently, by Issler et al.41 an increased incidence with normal average BMI. We also did not set a significant correlation between BMI > P85 and age or BMI > P85 and the 2013‑2017 group, although we noted a gradual increase of the ratio of patients above P85 for weight.
Etiology
Metabolic
The majority (54%) had a metabolic disturbance that was significantly related to family history of nephrolithiasis (p=0.001) and to dietary errors (p=0.032).
A single disturbance was identified in 56% of these patients. Overall, our most common metabolic disturbances were hypocitraturia (34%) and hypercalcuria (24%). Indeed, metabolic disturbances are the most recognized causes of nephrolithiasis worldwide.
Between 33 to 56% pediatric patients have an identifiable underlying metabolic risk factor18,39 and, in general, these are more likely in younger patients. The contribution of environmental factors, such as family diet patterns and dietary errors, are well recognized and our findings are in close accordance with previous studies27,29,42.
The relationship between metabolic disorders, dietary errors and family kidney stone history in our population probably reflect the family lifestyle pattern. On the other hand, we find that this higher risk is likely due to a combination of environmental exposures and genetic predisposition to calcium oxalate stone formation in families. Unlike the Sas DJ review5, our results did not show that metabolic causes were more prevalent in younger patients or in the more recent group.
Hypercalciuria and hypocitraturia are the most frequent metabolic causes of pediatric urolithiasis, reported between 7‑66%39,41,44 and 38‑63%36,41,44, respectively.
Moreover, Gürgöze et al36 identified hypocitraturia as the first metabolic cause (42%) compared to hypercalcuria (25%). Our findings are in agreement with these results (hypocitraturia 34%, hypercalcuria 24%).
Urinary tract infection
Infectious stones were found in 25% of our population and were more likely in patients under 2 years old (p=0.001), consistent with the 22% rate and younger age (median age 4 years versus 5.2 years metabolic group) observed by Issler et al.40. We observed a decrease in infectious causes throughout the study (p=0.043), which is in close accordance with the pediatric etiologic shift from predominantly infectious to metabolic causes15,16.
Idiopathic
In 14% we found no etiology. In the group study by Issler et al.41, 44% had no metabolic or infectious stones. Within this group, 19% had a primary renal structural malformation and 17% were premature and/or exposed to prolonged immobilisation. In our population, 13% were born prematurely and 10% had structural malformations, probably explaining our low rate of idiopathic cases. Nevertheless, none had a kidney stone analysis performed, which would had been helpful for a diagnosis. Sáez‑Torres C et al.31 suggested that low urinary volume might be the most important risk factor for stone formation in healthy individuals. Besides familial history and dietary errors, this was the most frequently recognized nephrolithiasis risk factor observed in this group (55%), although no statistically significant association was found. Indeed, in our entire sample, 37% had a low urinary volume.
Stone composition
In developed countries, calcium oxalate is the predominant stone constituent and the vast majority has mixed compositions41,42, similarly to our results. Moreover, bilateral stone disease was recognized in our population in 59%. In the Issler study group41, bilateral stones were found in 26% and were associated with metabolic causes. In our study, 58% of the population with metabolic disease had bilateral nephrolithiasis.
Furthermore, we found that struvite calculi were more frequent in children less than 2 years old (p=0.037) and correlated with infectious causes (p=0.033), regardless of gender. It may be explained by more common infections of the urinary tract in younger children and is in agreement with Kirejczyk JK findings44.
Outcome
Kidney stone disease has been associated with adulthood‑increased risk of coronary heart disease, CKD and hypertension45 and therefore a correct investigation and management are of higher importance. In our cohort, no cases developed hypertension. However, before the nephrolithiasis diagnosis, two patients followed for nephro‑urologic disease already presented hypertension and one CKD, representing an additional cardiovascular risk. One patient, with primary hyperoxaluria, progressed to CKD. In fact, inherited metabolic disorders such as primary hyperoxaluria are rare but important causes of CKD in children8 emphasizing the importance of a thorough metabolic evaluation.
Moreover, as metabolic disorders are more common in children, the risk of recurrence is higher than in adults15,41,42,46,47. We observed a 28% recurrence rate, but no significant specific cause was set. Among Tasian et al. group38, 24% developed symptomatic recurrent stones over the follow‑up, with 50% presenting symptomatic recurrence three years after the first episode.
The author also observed that completing a 24‑hour urine analysis was associated with a 60% decreased risk of recurrence, again enhancing the value of the metabolic investigation.
Limitations
The retrospective nature of this study is its primary limitation: first, complete metabolic work‑up (magnesuria and phosphaturia) was not systematically performed but this fact was also pointed out by Issler et al.41; secondly, dietary intakes were estimated and not evaluated through a validated questionnaire. Besides, this is not a population‑based study and our results may vary from the overall pediatric Portuguese nephrolithiasis epidemiology.
Finally, genetic studies were sporadic, explaining why only one patient from the hyperoxaluria group had a genetic confirmation, and this might be relevant in positive family history cases with no other identified risk factor.
CONCLUSION
In conclusion, nephrolithiasis referrals increased throughout the study period. Infectious causes predominate at younger ages and decreased throughout the study. Metabolic abnormalities were the most frequent cause. As they usually are responsible for nephrolithiasis appearance, a complete screening in children is of crucial importance. To prevent recurrence, renal scarring and cardiovascular events, educating and promoting a healthy life style is a primary priority.
Contributions of all authors
Joana V. Andrade: Bibliographic research, study design, data collection, statistical analysis of the results, article script and critical revision.
Sofia Bota: Bibliographic research, study design, data collection, statistical analysis of the results, article script and critical revision.
Telma Francisco; Bibliographic research, study design, data collection, statistical analysis of the results, article script and critical revision.
Raquel Santos: Bibliographic research, study design, data collection, statistical analysis of the results, article script and critical revision.
Gisela Neto: Bibliographic research, study design, data collection, statistical analysis of the results, article script and critical revision.
Margarida Abranches: Bibliographic research, study design, data collection, statistical analysis of the results, article script and critical revision.
References
1. Rizvi SA, Naqvi SA, Hussain Z, et al. Pediatric urolithiasis: developing nation perspectives. J Urol 2002;168:1522–1525 [ Links ]
2. Marx JA, Hockberger S, Walls RM. Rosens emergency medicine: concepts and clinical practice, 5th edition. Mosby, St. Louis, 2002 [ Links ]
3. Lande MB, Varade W, Erkan E, Niederbracht Y, Schwartz GJ. Role of urinary supersaturation in the evaluation of children with urolithiasis. Pediatr Nephrol 2005;20:491–494 [ Links ]
4. Kroovand RL. Pediatric urolithiasis. Urol Clin North Am 1997;24: 173–184 [ Links ]
5. Sas DJ. An update on the changing epidemiology and metabolic risk factors in pediatric kidney stone disease. Clin J Am Soc Nephrol 2011;6:2062–2068 [ Links ]
6. Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976‑1994. Kidney Int 2003;63:1817–1823 [ Links ]
7. Clayton DB, Pope JC. The increasing pediatric stone disease problem. Ther Adv Urol 2011;3:3–12 [ Links ]
8. Edvardsson V, Elidottir H, Indridason OS, Palsson R. High incidence of kidney stones in Icelandic children. Pediatr Nephrol 2005;20: 940–944 [ Links ]
9. Sas DJ, Hulsey TC, Shatat IF, Orak JK. Increasing incidence of kidney stones in children evaluated in the emergency department. J Pediatr 2010;157:132–137 [ Links ]
10. Bush NC. Epidemiological trends in pediatric urolithiasis. Editorial comment. J Urol 2010;184:1104–1105 [ Links ]
11. Routh JC, Graham DA, Nelson CP. Epidemiological trends in pediatric urolithiasis at United States freestanding pediatric hospitals. J Urol 2010;184:1100 [ Links ]
12. Baştuğ F, Düşünsel R. Pediatric urolithiasis: causative factors, diagnosis and medical management. Nat Rev Urol 2012;9:138–146 [ Links ]
13. Sarkissian A, Babloyan A, Arikyants N, Hesse A, Blau N, Leumann E. Pediatric urolithiasis in Armenia: a study of 198 patients observed from 1991 to 1999. Pediatr Nephrol 2001;16:728–732 [ Links ]
14. Basaklar AC, Kale N. Experience with childhood urolithiasis. Report of 196 cases. Br J Urol 1991;67:203–205 [ Links ]
15. Hoff WG. Aetiological factors in pediatric urolithiasis. Nephron Clin Pract 2004;98:c45–c48 [ Links ]
16. Cameron MA, Sakhaee K, Moe OW. Nephrolithiasis in children. Pediatr Nephrol 2005;20:1587–1592 [ Links ]
17. Garcia‑Nieto V, Navarro JF, Luis‑Yanes MI, Lopez‑Mendes M, Garcia‑rodriguez V. Hypercalciuria in pediatric patients with ureteropelvic junction obstruction is of genetic origin. Scand J Urol Nephrol 2007;41:144–148 [ Links ]
18. Naseri M, Varasteh AR, Alamdaran SA. Metabolic factors associated with urinary calculi in children. Iran J Kidney Dis 2010;4:32–38 [ Links ]
19. Trapote RA, Garagorri MAU, Arrieta MU, Monge TM, Lizarraga DA. Evaluation of renal stone disease: metabolic study. An Pediatr (Barc) 2004;61:418–427 [ Links ]
20. Nicoletta JA, Lande MB. Medical evaluation and treatment of urolithiasis. Pediatr Clin North Am 2006;53:479–491 [ Links ]
21. Ece A, Ozdemir E, Gürkan F, Dokucu AI, Akdeniz O. Characteristics of pediatric urolithiasis in south‑east Anatolia. Int J Urol 2000;7:330–334 [ Links ]
22. Ozokutan BH, Küçükaydin M, Gündüz Z, Kabaklioğlu M, Okur H, Turan C. Urolithiasis in childhood. Pediatr Surg Int 2000;16:60–63 [ Links ]
23. Battino BS, DeFOOR W, Coe F, et al. Metabolic evaluation of children with urolithiasis: are adult references for supersaturation appropriate? J Urol 2002;168:2568–2571 [ Links ]
24. Van Savage JG, Palanca LG, Andersen RD, Rao GS, Slaughenhoupt BL. Treatment of distal ureteral stones in children: similarities to the American urological association guidelines in adults. J Urol 2000;164:1089–1093 [ Links ]
25. Butani L, Kalia A. Idiopathic hypercalciuria in children–how valid are the existing diagnostic criteria? Pediatr Nephrol 2004;19:577–582 [ Links ]
26. Polito C, Signoriello G, Andreoli S, Manna A. Urinary urea excretion in idiopathic hypercalciuria of children. J Pediatr 2006;2:419–423 [ Links ]
27. Reddy ST, Wang CY, Sakhaee K, Brinkley L, Pak CY. Effect of low‑carbohydrate high‑protein diets on acid–base balance, stone‑forming propensity, and calcium metabolism. Am J Kidney Dis 2002;40:265–274 [ Links ]
28. Prezioso D, Strazzullo P, Lotti T, Bianchi G, Borghi L, Caione P, Carini M, Caudarella R, Gambaro G, Gelosa M, Guttilla A, Illiano E, Martino M, Meschi T, Messa P, Miano R, Napodano G, Nouvenne A, Rendina D, Rocco F, Rosa M, Sanseverino R, Salerno A, Spatafora S, Tasca A, Ticinesi A, Travaglini F, Trinchieri A, Vespasiani G, Zattoni F. Dietary treatment of urinary risk factors for renal stone formation. A review of CLU Working Group. Arch Ital Urol Androl 2015;87:105–120 [ Links ]
29. Taylor EN, Curhan GC. DASH‑Style diet associates with reduced risk for kidney stones. J Am Soc Nephrol 2009;20:2253–2259 [ Links ]
30. Borghi L, Schianchi T, Meschi T, Guerra A, Allegri F, Maggiore U, Novarini A. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 2002;346:77–84 [ Links ]
31. Saez‑Torres C, Grases F, Rodrigo D, Garcia‑Raja AM et al. Risk factors for urinary stones in healthy schoolchildren with and without a family history of nephrolithiasis. Pediatr Nephrol 2013;28:639–645 [ Links ]
32. Baştuğ F, Düşünsel R. Pediatric urolithiasis: causative factors, diagnosis and medical management. Nat Rev Urol 2012;9:138–146 [ Links ]
33. Li WM, Chou YH, Li CC, Liu CC, Huang SP, Wu WJ, Chen CW, Su CY, Lee MH, Wei YC, Huang CH. Association of body mass index and urine pH in patients with urolithiasis. Urol Res 2009;37:193–196 [ Links ]
34. Taylor EN, Curhan GC. Oxalate intake and the risk for nephrolithiasis. J Am Soc Nephrol 2007;18:2198–2204 [ Links ]
35. Edvardsson VO, Goldfarb DS, Lieske JC, Beara‑Lasic L, Anglani F, Milliner DS, Palsson R. Hereditary Causes of Kidney Stones and Chronic Kidney Disease. Pediatr Nephrol 2013;28:1923–1942 [ Links ]
36. Gürgöze MK, Sarı MY. Results of medical treatment and metabolic risk factors in children with urolithiasis. Pediatr Nephrol 2011;26:933–937 [ Links ]
37. Oguz U, Resorlu B, Unsal A. Metabolic evaluation of patients with urinary system stone disease: a research of pediatric and adult patients. Int Urol Nephrol 2014;46:329–334 [ Links ]
38. Tasian GE, Ross ME, Liahi S, Sas DJ et al. Annual incidence of nephrolithiasis among children and adults in South Carolina from 1997 to 2012. CJASN 2016;11:488–496 [ Links ]
39. Tasian GE, Kabarriti AE, Kalmus A, Furth SL. Kidney Stone Recurrence among Children and Adolescents. J Urol 2017;197:246–252 [ Links ]
40. Dwyer ME, Krambeck AE, Bergstralh EJ, Milliner DS et al. Temporal trends in incidence of kidney stones among children: a 25‑year population based study. J Urol 2012;188:247–252 [ Links ]
41. Issler N, Dufek S, Kleta R, vant Hoff W et al. Epidemiology of pediatric renal stone disease: a 22‑year single centre experience in the UK. BMC Nephrology 2017; 18:136 [ Links ]
42. VanDervoort K, Wiesen J, Frank R, Vento S et al. Urolithiasis in pediatric patients: A single center study of incidence, clinical presentation and outcome. J Urol 2007;177:2300–2305 [ Links ]
43. Spivacow FR, Negri AL, del Valle EE, Calvino I et al. Metabolic risk factors in children with kidney stone disease. Pediatr Nephrol. 2008;23:1129–1133 [ Links ]
44. Kirejczyk JK, Porowski T, Filonowicz R, Kazberuk A et al. An association between kidney stone composition and urinary metabolic disturbances in children. J Pediatr Urol 2014;10:130–135 [ Links ]
45. Bonzo JR, Tasian GE. The Emergence of Kidney Stone Disease During Childhood – Impact on Adults. Curr Urol Rep 2017;18:44 [ Links ]
46. Pietrow PK, Pope JC, Adams MC, Shyr Y, Brock JW. Clinical outcome of pediatric stone disease. J Urol 2002;167:670–673 [ Links ]
47. DeFoor W, Minevich E, Jackson E, et al. Urinary metabolic evaluation in solitary and recurrent stone forming children. J Urol 2008;179:2369–2372 [ Links ]
Joana Verdelho Andrade, MD
E‑mail: joanaverdelhoandrade@gmail.com
Disclosure of potential conflicts of interest: none declared.
Received for publication: Jul 1, 2018
Accepted in revised form: Sep 13, 2018














