Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.32 no.3 Lisboa set. 2018
ORIGINAL ARTICLE
Interventional nephrology – five years dealing with central stenosis: immediate and long-term results
Helena Pinto, Emanuel Ferreira, Nuno Afonso, Catarina Teixeira, Fátima Costa, Rui Alves
Centro Hospitalar e Universitário de Coimbra, Clínica Universitária de Nefrologia da FMUC
ABSTRACT
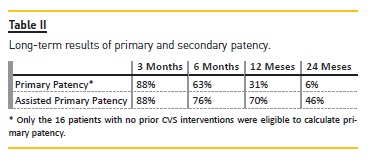
Introduction: Improved technique and materials have allowed us to prolong the life of hemodialysis vascular access using percutaneous transluminal balloon angioplasty (PTA). Central vein stenosis (CVS) can lead to arteriovenous access dysfunction or thrombosis. Our goal was to revise the outcomes of our institution, evaluating the immediate and long-term results in the endovascular treatment of CVS. Methods: We reviewed the data of all procedures performed in our center, Centro Hospitalar e Universitário de Coimbra, during a five-year period July 2009 and June 2014, selecting the cases that had a CVS diagnosis. We evaluated the immediate result and the existence of complications during the procedure. Long-term evaluation of PTA results of the 26 patients with a successful PTA was made through contact with the referring hospital or hemodialysis clinic. Primary and assisted access patencies were verified retrospectively at 3, 6, 12 and 24 months post-intervention. Results: Of the 31 patients in whom there were an intention to treat, in 5 the stenosis was in fact an occlusion and the guide wire could not be passed. The remaining 26 patients underwent PTA with improvement/resolution of the lesion. Consequently, we had an initial intervention success rate of 83.9%. Minor complications occurred in 2 patients. The long-term follow-up results were primary patency at 3, 6, 12 or 24 months of 88%, 63%, 31% and 6%, respectively, and assisted primary patency at 3, 6, 12 or 24 months of 88%, 76%, 70% and 46%, respectively. Conclusion: CVS is a common problem in hemodialysis patients. Our center results are consistent with current literature and demonstrate the benefit of PTA with excellent immediate success. However, the high recurrence rate of these stenoses requires in many cases multiple PTA, with low long-term primary patency.
Keywords: Arteriovenous Shunt, Surgical; Constriction, Pathologic; Renal Dialysis; Treatment Outcome; Vascular Patency.
INTRODUCTION
Central vein stenosis (CVS) is a common problem affecting dialysis patients and it is usually related to the vascular trauma associated with the previous or current presence of a central venous catheter (CVC) or even due to high flow fistulas, occurring at sites of turbulence. Other causes of growing importance are the presence of other endovascular devices, such as pacemaker wires or peripherally inserted central catheters.1-3
CVS can be asymptomatic and for this reason its true prevalence in hemodialysis patients is unknown. The majority of studies are limited to symptomatic patients and have reported a CVS prevalence of 19%-41%. It can manifest with progressive arm swelling, sometimes extended to the shoulder, neck, face and breast, and accompanied by pain or discomfort. In extreme situations, CVS can lead to respiratory distress caused by laryngeal edema, pleural effusion, chest swelling or even neurological symptoms. In addition to swelling, the main signs of an important CVS are skin discoloration, lymphatic blistering or weeping, stasis ulcers, skin and soft tissue infection and necrosis, nonhealing wounds or incisions, venous collaterals or phlebitis. CVS seldom leads to inadequate dialysis and arteriovenous (AV) access thrombosis.4-6
When the patient has an ipsilateral functioning dialysis access, it can increase the blood flow beyond the outflow venous capacity, resulting in venous hypertension and development of collateral veins. The development of collateral veins may temporarily lessen the symptoms and allow for the use of the access.5
Angiography is the gold standard for the diagnosis of CVS. NFK KDOQI (National Kidney Foundation Kidney Disease Outcomes Quality Initiative) guidelines recommend percutaneous transluminal balloon angioplasty(PTA), with or without stent placement, as the preferred approach to CVS.7 PTA has excellent initial results, but poor long-term efficacy, frequently needing multiple procedures in order to preserve a functioning access.
The purpose of this study was to evaluate our centers immediate and long-term results in the endovascular treatment of CVS.
PATIENTS AND METHODS
We retrospectively reviewed the data of all procedures performed in our center, Centro Hospitalar e Universitário de Coimbra, during the five-year period July 2009 and June 2014, selecting from reports the cases that had a diagnosis of CVS.
All the interventions were performed by the same lead operator, a nephrologist with experience in the field of vascular access.
From a total of 238 interventions, 36 had a significant CVS. Significant CVS was defined as an occlusion of at least 50% of the lumen in a central vein with the presence of collateral circulation. In 5 of them, the angioplasty could not be performed because there were no balloons available; consequently, those cases were excluded from our study. In another 5 patients, the stenosis was critical and it was not possible to overcome it with the guide wire; those cases were reported as technical failures. In the 26 remaining cases, technical aspects of the procedure, the PTA final result and the existence of complications were recorded. The reason for referral, patient vascular access at the time of intervention and the existence of previous PTA of the CVS were also analyzed.
We did not perform a single stent implantation during the interventions. In our center, stent usage is limited to extreme situations of early stenosis (<3months), usually in patients without vascular patrimony for construction of other arteriovenous access. Of the 26 patients undergoing PTA, long-term evaluation of PTA results was made through contact with the referring hospital or hemodialysis clinic. Primary and assisted primary patencies were verified retrospectively at 3, 6, 12 and 24 months after the intervention.
Success and Patency Definition
Anatomical technical success was defined as complete resolution of the stenosis after PTA or presence of residual stenosis of less than 30% after PTA. Longterm success rates were divided into primary (unassisted) patency, primary assisted patency and secondary patency. Primary patency was defined as the time between the intervention and access thrombosis, or a second intervention needed to maintain patency.
Assisted primary patency was defined as the interval between the intervention and access thrombosis, or a surgical intervention not involving the treated lesion from the access circuit. Secondary patency was defined as the time between the intervention until the access was surgically declotted (thrombolysis and percutaneous thrombectomy were considered secondary patency), revised or abandoned.8
RESULTS
Population Characterization
The main patient characteristics are presented in Table I.
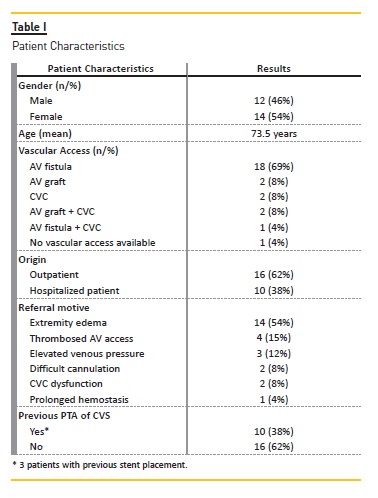
Procedure description
From the 26 cases, in the majority (23 patients) vascular access puncture or CVC was used for the procedure.
However, in 3 patients the femoral vein was used: two patients because the stenosis was not possible to overcome anterogradely and it was possibly retrogradely; one patient without vascular access (a patient with serious vascular access problems and in whom CVC was removed due to CVC sepsis). The majority of cases (75%) required only local anesthetic; the remaining 25% required conscious sedation with midazolam or intravenous morphine. High-pressure balloons with diameters between 8 and 16mm were used for angioplasty.
In two cases the access was thrombosed at the time of the intervention, requiring also thrombectomy and thrombolysis. A second stenosis in the drainage vein was present in two patients and a stenosis intra-graft was present in another one.
Immediate Success Results
Of the 31 patients in whom there was an intention to treat, in 5 patients, the stenosis was in fact an occlusion and the guide wire could not be passed. The remaining 26 patients underwent PTA with improvement/resolution of the lesion. Consequently, we had an initial intervention success rate of 83.9%.
Anatomical technical success was achieved in 22 patients (84.6%). The remaining 4 patients had an improvement of the lesion, but with a residual stenosis of 30% to 60%. In two cases there was no larger balloon diameter available and in the other 2 cases the PTA allowed placement of CVC.
Minor complications occurred in 2 patients, resulting in 1 prothesis rupture and 1 venous rupture, both with limited contrast extravasation and resolved with endovascular hemostasis, by inflating the angioplasty balloon at a low pressure.
Clinical signs of success, such as the appearance of a continuous palpable thrill (no pulse), lessening of the extremity tension, disappearance of collateral veins or pulsatility were present in all cases. However, in 2 patients, the arteriovenous access was abandoned and a CVC was placed after the PTA.
Long-Term Follow-up Results
Median follow-up time was 16.96 months. Two patients died during the follow-up. The causes were not related to the vascular access, which remained active until the death of both patients.
The long-term follow-up results are presented in Table II.
DISCUSSION
A higher risk of CVS has been associated with multiple CVC placements and longer catheter dwell times1,9.
Many authors have shown that placement of subclavian catheters produce a higher incidence of CVS than internal jugular catheters10-12. There is also evidence that internal jugular catheters produce more CVS when placed on the left side.13
The pathophysiology is unknown but several mechanisms could be involved in the development of CVS, such as trauma induced by intravascular devices, causing intimal hyperplasia and inflammatory response within the vessel wall, and the increase in blood flow turbulence. This inflammatory response leads to platelet deposition and venous wall thickening.14
Recently, angiography has become the first-choice treatment for CVS. Studies evaluating PTA are small and retrospective, although they all report a high technical immediate success, ranging from 70 to 95 percent.11,15-18 One of the main reason for technical failure is the elastic property of some of these lesions and many authors believe that stent placement is the best option in those cases.19,20
Some studies, as this one, report the primary and assisted primary patency rates for PTA treatment. Our results are comparable to those in the present literature that account for six-month primary and cumulative patency rates of 23 to 63 percent and 29 to 100 percent, respectively, and for 12-month primary patency rates of 12 to 50 percent and cumulative rates of 13 and 100 percent.3,11,16-18,21-23
The majority of authors believe that in vascular access stenosis, especially in CVS, the indication to treat should not be based only on anatomic criteria such as >50% decrease in intraluminal diameter. Clinical and physiological abnormalities should guide which CVS have to be treated. Development of collateral veins can improve symptoms, so an initial conservative approach is recommended. There has been evidence that PTA in asymptomatic central stenosis accelerates the stenosis progression leading to its rapid recurrence; thus it is not indicated.5,24
In fact, one major problem of CVS is the restenosis after PTA, demanding repeated PTA in order to preserve the access. The large majority of studies advocate the use of stents and PTA with a drug-eluted balloon in case of rapid recurrence or an important elastic recoil.20,25,26
Our study has several limitations: it is a retrospective study, the sample is small and the referral motive, as well as the subsequent monitorization of the vascular access, depends on the clinical judgment of different observers and on the experience and protocols of different dialysis units.
CONCLUSION
CVS is a common in hemodialysis patients and can lead to the development of symptoms and/or access dysfunction. PTA is a minimal-invasive technique that has a high rate of immediate success but the long-term primary patency is not optimal. Prevention of CVS development is important, especially by avoiding unnecessary CVC. Careful attention should be paid to the selection of which CVS to treat. Early procedures can, in fact, accelerate stenosis progression. The treatment of asymptomatic CVS is not recommended.
Our center results are consistent with those seen in current literature. PTA is a safe procedure, allowing the maintenance of an active access, although in some cases, multiple PTA is required due to rapid restenosis.
References
1. MacRae JM, Ahmed A, Johnson N, Levin A, Kiaii M. Central vein stenosis: a common problem in patients on hemodialysis. ASAIO journal. 2005;51(1):77-81. [ Links ]
2. Lopera G, Beathard GA, Exaire J, Carrillo R. Cardiac implantable electronic devices in end-stage renal disease patients: preservation of central venous circulation. J. Interv. Card. Electrophysiol. 2012;34(1):101-4. [ Links ]
3. Dammers R, de Haan MW, Planken NR, van der Sande FM, Tordoir JH. Central vein obstruction in hemodialysis patients: results of radiological and surgical intervention. Eur J Vasc Endovasc Surg. 2003;26(3):317-21. [ Links ]
4. Barrett N, Spencer S, McIvor J, Brown EA. Subclavian stenosis: a major complication of subclavian dialysis catheters. Nephrol Dial Transplant. 1988;3(4):423-5. [ Links ]
5. Agarwal AK. Central vein stenosis. Am J Kidney Dis. 2013;61(6):1001-15. [ Links ]
6. Dolmatch BL, Gurley JC, Baskin KM, Nikolic B, Lawson JH, Shenoy S, et al. Society of Interventional Radiology Reporting Standards for Thoracic Central Vein Obstruction: Endorsed by the American Society of Diagnostic and Interventional Nephrology (ASDIN), British Society of Interventional Radiology (BSIR), Canadian Interventional Radiology Association (CIRA), Heart Rhythm Society (HRS), Indian Society of Vascular and Interventional Radiology (ISVIR), Vascular Access Society of the Americas (VASA), and Vascular Access Society of Britain and Ireland (VASBI). J Vasc Interv Radiol. 2018;29(4):454-60e3. [ Links ]
7. Hemodialysis Adequacy Work G. Clinical practice guidelines for hemodialysis adequacy, update 2006. Am J Kidney Dis. 2006;48(1):S2-90. [ Links ]
8. Gray RJ, Sacks D, Martin LG, Trerotola SO, Society of Interventional Radiology Technology Assessment C. Reporting standards for percutaneous interventions in dialysis access. J Vasc Interv Radiol. 2003;14(9 Pt 2):S433-42. [ Links ]
9. Vanherweghem JL, Yassine T, Goldman M, Vandenbosch G, Delcour C, Struyven J, et al. Subclavian vein thrombosis: a frequent complication of subclavian vein cannulation for hemodialysis. Clin Nephrol. 1986;26(5):235-8. [ Links ]
10. Schillinger F, Schillinger D, Montagnac R, Milcent T. Post catheterisation vein stenosis in haemodialysis: comparative angiographic study of 50 subclavian and 50 internal jugular accesses. Nephrol Dial Transplant. 1991;6(10):722-4. [ Links ]
11. Maskova J, Komarkova J, Kivanek J, Danes J, Slavikova M. Endovascular treatment of central vein stenoses and/or occlusions in hemodialysis patients. Cardiovasc Intervent Radiol. 2003;26(1):27-30. [ Links ]
12. Schwab SJ, Quarles LD, Middleton JP, Cohan RH, Saeed M, Dennis VW. Hemodialysisassociated subclavian vein stenosis. Kidney Int. 1988;33(6):1156-9. [ Links ]
13. Schon D, Whittman D. Managing the complications of long-term tunneled dialysis catheters. Semin Dial. 2003;16(4):314-22. [ Links ]
14. Forauer AR, Theoharis C. Histologic changes in the human vein wall adjacent to indwelling central venous catheters. J Vasc Interv Radiol. 2003;14(9):1163-8. [ Links ]
15. Beathard GA. Percutaneous transvenous angioplasty in the treatment of vascular access stenosis. Kidney Int. 1992;42(6):1390-7. [ Links ]
16. Glanz S, Gordon DH, Lipkowitz GS, Butt KM, Hong J, Sclafani SJ. Axillary and subclavian vein stenosis: percutaneous angioplasty. Radiology. 1988;168(2):371-3. [ Links ]
17. Asif A, Salman L, Carrillo RG, Garisto JD, Lopera G, Barakat U, et al. Patency rates for angioplasty in the treatment of pacemaker-induced central venous stenosis in hemodialysis patients: results of a multi-center study. Semin Dial. 2009;22(6):671-6. [ Links ]
18. Rajan DK, Chennepragada SM, Lok CE, Beecroft JR, Tan KT, Hayeems E, et al. Patency of endovascular treatment for central venous stenosis: is there a difference between dialysis fistulas and grafts? J Vasc Interv Radiol. 2007;18(3):353-9. [ Links ]
19. Kovalik EC, Newman GE, Suhocki P, Knelson M, Schwab SJ. Correction of central venous stenoses: use of angioplasty and vascular Wallstents. Kidney Int. 1994;45(4):1177-81. [ Links ]
20. Vogel PM, Parise C. SMART stent for salvage of hemodialysis access grafts. J Vasc Interv Radiol. 2004;15(10):1051-60. [ Links ]
21. Lumsden AB, MacDonald MJ, Isiklar H, Martin LG, Kikeri D, Harker LA, et al. Central venous stenosis in the hemodialysis patient: incidence and efficacy of endovascular treatment. Cardiovascular surgery. 1997;5(5):504-9. [ Links ]
22. Quinn SF, Schuman ES, Demlow TA, Standage BA, Ragsdale JW, Green GS, et al. Percutaneous transluminal angioplasty versus endovascular stent placement in the treatment of venous stenoses in patients undergoing hemodialysis: intermediate results. Cardiovascular surgery. 1995;6(6):851-5. [ Links ]
23. Surowiec SM, Fegley AJ, Tanski WJ, Sivamurthy N, Illig KA, Lee DE, et al. Endovascular management of central venous stenoses in the hemodialysis patient: results of percutaneous therapy. Vascular and endovascular surgery. 2004;38(4):349-54. [ Links ]
24. Levit RD, Cohen RM, Kwak A, Shlansky-Goldberg RD, Clark TW, Patel AA, et al. Asymptomatic central venous stenosis in hemodialysis patients. Radiology. 2006;238(3):1051-6. [ Links ]
25. Massmann A, Fries P, Obst-Gleditsch K, Minko P, Shayesteh-Kheslat R, Buecker A. Paclitaxel-coated balloon angioplasty for symptomatic central vein restenosis in patients with hemodialysis fistulas. J Endovasc Ther. 2015;22(1):74-9. [ Links ]
26. Maya ID, Saddekni S, Allon M. Treatment of refractory central vein stenosis in hemodialysis patients with stents. Semin Dial. 2007;20(1):78-82. [ Links ]
Helena Pinto, MD
Centro Hospitalar e Universitário de Coimbra
Clínica Universitária de Nefrologia da FMUC
Praceta Prof. Mota Pinto, 3000-075 Coimbra, Portugal
E-mail: helenasofiapinto@gmail.com
Disclosure of potential conflicts of interest: none declared
Received for publication: Jan 14, 2018
Accepted in revised form: Apr 12, 2018














