Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.32 no.3 Lisboa set. 2018
PERSPECTIVE
Transitions of care management in CKD: critical thinking and improving strategies
Isabel M Correia1, Anabela S Rodrigues1,2
1 Unit for Multidisciplinary Research in Biomedicine / ICBAS‑UP
2 Nephrology Department, Centro Hospitalar do Porto – Hospital de Santo António
ABSTRACT
Chronic kidney disease (CKD) has a high clinical and socioeconomic impact and is often associated with multimorbidity. Improved treatment has allowed an increase in patient survival, but patient life expectancy remainslimited. The disease course has a continuum of lesion, stage and treatment transitions. The focus is often placed on treatment modality, disregarding the course of a CKD patients disease. In addition, patient management in transitions of modalities of renal replacement therapy (RRT) can also be a vector for improving clinical outcomes.
The transition between different types of CKD treatment and the transition of care from paediatric to adult team are critical processes throughout the life of a CKD patient. In the therapeutic transition, there is the need to identify better predictors of success in allocating patients with stage 5 CKD to their first dialytic modality in.
There is a risk of early mortality in the induction period of dialysis, particularly of the elderly in extracorporeal dialysis regimens. Doubt remains in decision making about the ideal timing to establish the transition to renal replacement therapy and its most appropriate type. Transfer between dialytic modalities also calls for opportune and integrated policies protecting vascular resources. Renal transplantation is considered the optimal renal replacement therapy; however, transplant failure or the side effects of immunosuppression are threats to consider, which may redirect these patients back to dialysis and involves a re‑evaluation of the patients status. Also, end‑of‑life care and decision making between initiating renal replacement therapy or maintaining conservative management are a challenge in the elderly.
This review identifies the main challenges in these transitional processes, raising awareness of areas in need of improvement in patient care. The aim should be to achieve a more comprehensive and appropriate health management than a limited focus on CKD modality treatment.
Keywords: Chronic kidney failure; Renal replacement therapy; Conservative non‑dialytic treatment; Quality of life; Transitional care; Continuity of patient care.
INTRODUCTION
Chronic kidney disease (CKD) is an important public health problem, with increasing prevalence and health costs, and associated with poor clinical outcomes, such as decreased quality of life, progression to renal failure, all‑cause and cardiovascular mortality1-4.
A global improvement in treatment and outcomes of CKD has been achieved but the threat of patient limited survival with high burden of care and costs still looms.
Several insufficiencies and problems are identified in CKD stages and transition process, as shown in Tables I, II, III, IV, V, VI, VII.


Quality control processes are often focused on controversial indicators lacking patients perceptions of quality and an integrated approach to the modalities of treatment. Further, the focus is often placed on treatment modality, disregarding the course of a CKD patients disease.
We propose that the management of transitions in CKD treatments should also be included in the quality improvement processes of nephrology departments as a vector for improving clinical outcomes. The aim of this review is to present a critical appraisal of these opportunities for improvement over the course of a CKD patients disease.
CHRONIC KIDNEY DISEASE AND RENAL REPLACEMENT THERAPY OPTIONS
Prevention of acute renal lesions is mandatory and there should be a task force in public health and hospitals aiming toward nephroprotection. The progression of CKD may not have a steady linear decline and show a heterogeneous trajectory5. Estimated glomerular filtration rate (eGFR) trajectories are independent predictors of advanced stage CKD, with clinical implications for the timing of RRT initiation5,6. In patients with advanced stage CKD, fragmentation of care, inadequate vascular accesses, lack of focus on the management of comorbidities and absence of preventive care are obstacles that result in poor clinical outcomes and higher costs7. Iatrogenic episodes leading to acute kidney injury, hospitalization or crisis with loss of functional abilities are frequent. A disease management program aims to improve results with cost savings7, with benefits in the progression of CKD, risk of renal failure and quality of life3. In such a program, multidisciplinary collaboration is fundamental7. The nephrologist plays a leading role, ensuring coordinated care7. The nurse is essential in the integration of care, in follow‑up and as a centralizing care member among different physicians, other health professionals and the patient7.
A management plan initiated in stage 3‑4 CKD and/or a specific low clearance consultation in interaction with the primary care physician are examples of transition care optimization measures. This plan should include:
1. Flagging up of clinical and social risk situations and timely referral to multidisciplinary team members.
2. Systematic nephroprotection (anticipation of clinical risk, patient and family education, eviction of drug redundancy and adverse effects, nephrotoxic eviction, eviction of puncture of vascular accesses in the non‑dominant limb for preservation of vascular patrimony).
3. Efficient post‑discharge hospital communication with improved rehabilitation care.
4. Use of non‑presential contact to complement support to patients and families in the post‑hospitalization phase.
5. Assessment of patient and family expectations about the disease and its treatment modalities.
6. Evaluation of fragilities and comorbidities with prognostic impact that imply limited life expectancy / quality of life under dialysis and equation of non‑dialytic support treatment.
7. Optimization of palliative care measures when indicated.
Presently, the financial constraint of the country has limited this intervention; however a long‑range vision may show that investment in this critical transition phase can save costs related to the inexistence of this management process.
RRT therapies in end‑stage renal disease include renal transplantation, haemodialysis (HD) and peritoneal dialysis (PD)8. Upon reaching stage 4 CKD, patients should discuss RRT options with the nephrologist for a timely preparation8, in full respect of the informed consent process of choice of therapies. There is a recognized higher risk of death in the first 3‑6 months after initiation of RRT, whether dialysis or transplantation is involved9.
Renal transplantation allows longer survival and better quality of life than dialysis, and pre‑emptive transplantation is preferable8. However, in the present system of renal graft allocation (from deceased donors), the previous time on dialysis is scored to mitigate the effect of pre‑transplantation waiting time of many dialysis patients. Therefore, to apply the strategy of pre‑emptive renal transplantation more effectively, a task force on living organ donation must be put forward, both in nephrology and in society. According to KDIGO guidelines10, pre‑emptive transplantation should be considered at eGFR <20ml/min/1.73m2 with evidence of progressive and irreversible CKD in the last 6‑12 months.
The clinical guidelines10 suggest that dialysis should be initiated in the presence of signs or symptoms attributable to renal failure, inability to control volume status or blood pressure (BP), progressive deterioration of nutritional status refractory to dietary intervention or cognitive impairment. This often occurs with eGFR of 5 to 10ml/min/1.73m210; however, the decision to initiate dialysis should not depend on an isolated numerical value11. For patients who choose HD, arteriovenous fistula (AVF) is the vascular access preferred due to the lower rates of infection, thrombosis and interventions to maintain patency8. The benefit associated with longer or more frequent sessions than in conventional HD has resulted in increased interest in nocturnal HD, in‑centre or at home8,12. PD is a good option for motivated adult patients capable of self‑care and also those of paediatric age8 or when assisted PD is viable. Desirably, domiciliary therapies should be chosen whenever possible. Conservative non‑dialytic treatment (CNT) should be an option for elderly patients10 with expected shorter survival due to severe comorbidities.
In the treatment trajectory (Fig. 1), changes in therapy are common13 and should be anticipated13,14 because the transition periods are associated with a state of patient greater vulnerability, a very high rate of adverse events and critical decision making15. However not all causes of transfer mean the same in terms of impact in global patient survival and more investigation should be focused on the best track of transfers to protect the patient.
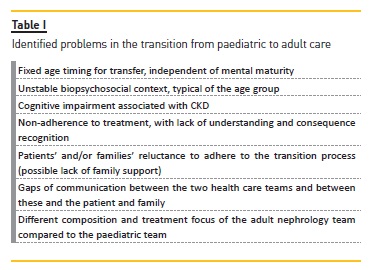
TRANSITION FROM PAEDIATRIC TO ADULT CARE
Transition from paediatric to adult care is a period of great anxiety for young patients and their parents16.
The transition in this age group is associated with poor outcomes, difficulties in self‑management of CKD, decreased therapeutic compliance and increased avoidable hospitalizations16-18. In renal transplant adolescents and young adults, there is also the risk of acute rejection, chronic graft loss or dysfunction due to poor compliance with immunosuppression16,19, which is independent of the transplantation age20. Non‑adherence to medical treatment is the main cause of graft loss in adolescents, due to lack of understanding and consequence recognition20-22. The cognitive impairment and delayed cognitive development associated with CKD, although apparently subtle, may be an important contribution to poor adherence20.
These outcomes are not only related to the transition itself, but are mainly a reflection of the unstable biopsychosocial context of this age group16,22, between 14‑24 years, who are still in the brain development and maturation process16,19,23. Patients transferred at an older age appear to have better outcomes20,22. There might also be a reluctance of patients and parents to leave paediatric care16.
In this transition, the patient is expected to be progressively more prepared to assume responsibility for the management of CKD16,18,23, with its medications, procedures and nutritional requirements19.
In‑centre HD patients appear to have worse CKD self‑management abilities than those in dialysis home modalities19, but individualization is mandatory, taking into consideration patient mental ability and family context.
A paediatric unit is multidisciplinary, has psychosocial support, fewer patients, is more family oriented, considers growth and development issues and has more experience in congenital anomalies of the kidney and urinary tract and other nephropathies that most commonly cause CKD in children and adolescents19,24,25.
Sometimes follow‑up in adult care has less support in their teams, is more individualized24 and requires greater autonomy, which is not possessed by the paediatric age group shortly before transference16. Gaps of communication between the two teams or these and the patient and family are another barrier to successful transition18.
In the face of the problems identified in this transition, summarized in table I, the implementation of a transition program between paediatric and adult care is identified as a major measure of optimization. This transition program consists of a well‑structured process, involving a multidisciplinary team of paediatric and adult nephrologists, leading to improved long‑term outcomes16. Based on the clinical recommendations of the International Society of Nephrology and International Paediatric Nephrology Association23 these programs should include:
1. A health professional who oversees long‑ term planning and coordinates the remaining members of the team23.
2. Early onset of the transition process, in Paediatric Nephrology with continuity and follow‑up by the reference physician in adult Nephrology, until acquisition of self‑management skills of CKD is optimized23.
3. Active involvement of the patient and family23.
4. Transfer during a period of stability, with effective communication ensured between the paediatric and adult teams16,23.
5. Promoting access to peer support structures in the preparation19,23 and, above all, after transfer to an adult unit26.
6. Annual assessment of the patients ability for transition and self‑management
skills of CKD19, so that it is possible to individualize the timing of transition, depending on the patients capabilities and maturity23, and not at a fixed age time.
7. Interventions for the education and management of several areas of the disease (nutrition, BP, laboratory results, drug management) whenever necessary19.
A transition clinic, where the young person is seen jointly by paediatric and adult nephrologists, shortly before transfer, is considered an ideal transition method23.
T RANSITION TO PRE‑EMPTIVE TRANSPLANTATION
Renal transplantation is considered the best RRT in eligible patients and is responsible for a greater improved quality of life than dialysis3,27,28. A renal transplant patient has longer life expectancy, feels better, has more ability to continue working, and is less likely to be hospitalized3. Living donor transplantation has better outcomes, can be scheduled in time and be faster than deceased donor transplantation3. Renal transplantation is also the most cost‑effective treatment, although most health expenditures in RRT are directed at dialysis, because of the limitation of available organs4.
Preparation for renal transplantation is best achieved when there is a clinically controlled progression between stages 4 and 5 of CKD11. Despite this, many patients achieve advanced stage CKD without the desirable preparation11.
Pre‑emptive transplantation is the preferred RRT and avoids dialysis, which is in line with the current recognition that reducing the dialysis period has advantages in quality of life, clinical outcomes and health costs27. Patients may experience renal transplantation in a very different way, depending on whether they have undergone a previous dialysis period or have gone through surgery with a functioning kidney27. Patients undergoing pre‑emptive transplantation may not be aware of the benefits of transplantation as compared to dialysis and can also experience the suffering of transplantation, as a result of surgery or immunosuppressive therapy27. Compared to patients with previous dialysis, those undergoing pre‑emptive transplantation are more likely to have a lower perceived quality of life after transplantation and more difficulty adapting to their new condition, although they generally have a better level of physical health27.
The value of renal transplantation can vary considerably, also among patients who performed it pre‑emptively, with descriptions of patients who considered it responsible for a deterioration of their well‑being27.
The patients perception of the impact of this RRT strategy may be the result of the global variability of the pre‑emptive transplant practice and this is important information, as it may have consequences for compliance, graft and patient survival27.
Currently, only 2.6% of patients receive pre‑emptive renal transplantation3, which implies an available living donor, generally a patients relative. Nevertheless, pre‑emptive transplantation has a lower cost, is associated with longer graft survival and a lower rate of acute organ rejection, which gives the patient a survival advantage compared to transplantation after a previous dialysis period3.
Faced with these discrepancies (summarized in table II), the implementation of an educational program with coordination of care will optimize the process. It should integrate:
1. Patient follow‑up, favouring a clinically controlled progression of CKD between stages 4 and 5.
2. Promotion of debate with the patient and his family about the appropriate RRT options for his particular situation, including repeated clarification of the advantages and commitments inherent for the patient in renal transplantation, and particularly pre‑emptive transplantation3,27.
3. Patient follow‑up after the option for pre‑emptive transplantation, with focus on compliance promotion, education for nephroprotection and prevention of CKD in the graft, in order to prevent its premature failure27.
4. Promotion of live donor transplantation3, social valuation of organ donation and protection of the donor.
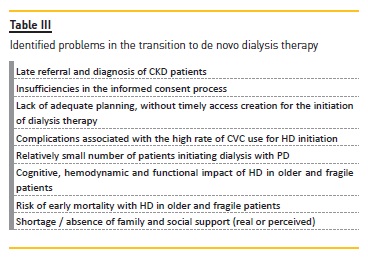
TRANSITION TO DE NOVO DIALYSIS THERAPY
The onset of de novo dialysis, previously prepared and planned, is associated with better short‑and long‑term outcomes29. However, the onset of dialysis is often unplanned and complicated by temporary vascular access and sometimes without the possibility of providing the patient with their modality or location of choice30.
This process of dialysis access creation, seldom optimal, lowers the quality of care and increases its cost3. Studies on patients opinions show that up to 30% would opt for PD4. Moreover, participation in some educational programs on dialysis results in a greater probability of PD, a lower probability of HD with catheter and lower mortality in the first 90 days after initiating dialysis3.
PD has shown recent outcome improvement and should be made available to patients with the ability to perform the procedure11. Also, in patients who are unable to perform auto‑dialysis should be assisted in the modality they opt, as for example assisted PD. Compared to in‑centre HD, PD as the initial modality of RRT is associated with preservation of RRF, better survival in the first one to two years, is more cost‑effective and allows patients greater rehabilitation[31]. Therefore, there is an effort to increase the choice for this home modality31. However, a more focused peritoneal access implantation program is needed to support this strategy and avoid early access complications that threaten immature PD programmes.
Patients who receive comprehensive education on CKD more often choose PD or home HD11,32. The obstacles to these options are patients and families disinterest and lack of social support32. The choice of home HD is associated with younger age, job maintenance and absence of language barrier32. In fact, home dialysis is a cost‑effective RRT, associated with better quality of life compared to in‑centre HD, but it requires the patients ability to perform dialysis himself32.
It is fundamental to adequately prepare the patient with CKD, regardless of the dialytic modality of choice, when reaching advanced stage CKD11. The HD option has challenges, such as several weekly treatments, risk of rapid fluid and electrolyte shifts, complications of comorbidities, vascular access and reduced life expectancy33 and often unprevented acute kidney injury that accelerates the trajectory of renal failure. The onset of HD is unscheduled in up to 50% of patients, mainly due to late diagnosis or referral34. This insufficient preparation results in a low frequency of AVF, increasing HD with central venous catheter (CVC), which results in an increase in catheter‑associated infections, hospitalizations, morbidity, mortality and higher costs3,11,30,34. A further negative effect is the lower number of patients initiating PD11. Fluck29 advocates the use of continuous ambulatory PD as transient dialysis therapy in the context of unplanned initiation of dialysis.
In the HD option, PD would be a resource option during the maturation of AVF, which would involve acute placement of peritoneal catheter (possible obstacle)29,30. The authors mentioned that peritoneal catheter placement can be readily available with initial safety rates comparable to those of temporary CVC29, with lower risk of bacteraemia, sepsis and the hemodynamic instability associated with extracorporeal dialysis. It has been demonstrated that the coordination of pre‑dialysis care increases the use of surgical vascular access in HD, improves bone mineral metabolism parameters at dialysis initiation and reduces orbidity and mortality after its onset3,35. Reduction of total mortality, cardiovascular mortality and early mortality are critical in this transition phase15; hence the importance of investigating its predictive factors.
Faced with the problems summarized in Table III, the main areas for improvement are:
1. Support of a multidisciplinary team in pre‑dialysis34 and promotion of the appropriate management of CKD, aiming for outcomes including patient reported health and well‑being, burden of care, disutility of care, such as recommended by the International Consortium for Health Outcomes36,37.
2. Coordination of care3,35, in order to create the best vascular access or peritoneal access in a timely fashion.
3. Inclusion of an element (such as a nurse) in the multidisciplinary team capable of promoting patient education, in order to streamline subsequent transitions, particularly to more autonomous RRT modalities34.
4. Psychological support, integrated with the rest of the team, to facilitate the patients process of adaptation to his new medical condition.
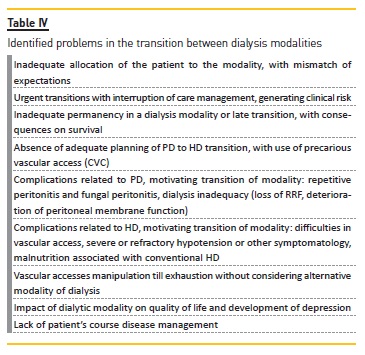
TRANSITION BETWEEN DIALYSIS MODALITIES
Patients often shift from dialysis modalities, so it is fundamental to investigate which is the best transition process in the course of a CKD patients disease, with better survival and less comorbidity.
Transitions from PD to HD are more frequent because the technique relies on a single access and the capacity of the patient (if not assisted). Transitions from HD to PD are less frequent because the vascular resources are progressively used with multiple procedures and, in the absence of capacity, the technique remains assisted in‑centre HD. This isnt a drawback ofPD in the course of patients disease since an elective and adequate transition doesnt reduce the patients survival38,39.
Patients who adequately change modalities can benefit, in due time, from the advantages of each one.
Conversely, persisting in one modality may be associated with decreased survival. Other than survival, clinical results should also value the preservation of RRF, of vascular capital for future life and the best rehabilitation and self‑determination.
PD patients move to HD for several reasons, such as recurrent infections, inadequate dialysis due to loss of RRF, and deterioration of peritoneal membrane function40.
This transition should be anticipated and planned, since unscheduled HD start is associated with increased risk of death41. It is important that these patients already have a mature AVF40. On the other hand, PD to HD transition can be very troublesome, especially when there is acute, often unpredictable, PD failure30, such as after refractory peritoneal infection or after abdominal emergency surgery, what usually implies CVC30,40. The timely prediction of PD failure is not easy and is a concern for nephrologists because it takes time to create and maturate an HD access29,41.
Besides the type of vascular access, the timing of PD to HD transition may have different impact: early transitions from infection episodes rarely impact overall survival, but late transitions associated with metabolic complications or inadequacy may have a negative impact per se.
According to Nadeau‑Fredette et al.42, patient and technique survival of those on home HD previously submitted to PD is similar to those on home HD without previous PD. These results favour the home‑integrated dialysis model and the PD to home HD transition42.
HD to PD transition may be associated not only with late referral and lack of education in pre‑dialysis, but may also be the result of HD related complications, such as difficulties in vascular access, severe or refractory hypotension31.
According to Nessim et al.31, patients who move from HD to PD in the first year of dialysis have a higher risk of death and PD failure than those who are on PD since the onset of RRT, with time on HD not affecting PD failure. This may be due to the more rapid loss of RRF in the initial period of HD, greater difficulty in adapting to PDs self‑management after a period of dependence of in‑centre HD or by the presence of more severe pathology in the group of patients with an initial HD period31.
Shrestha et al43 evaluated the quality of life of patients on HD, continuous ambulatory PD and CNT, and found that PD patients have a better quality of life, especially in the area of mental health, possibly because they perceive a greater degree of independence.
Although overall quality of life is considered to be superior in patients on PD than on HD, risks (peritonitis, catheter obstruction, sclerosing peritonitis versus access infection and sepsis, hemodynamic intolerance and cerebrovascular events) and the cost‑effectiveness of each modality should be considered before the option for one of them43. Overall, patients survival on PD is higher in the early post‑induction years and is similar in the long run, even showing a five‑year advantage after starting dialysis in the 2017 United States Renal Data System report44.
Compared to the general population, depression and decreased quality of life are prevalent among patients on HD35. Concerning dialytic home modalities, patients on continuous ambulatory PD have higher levels of depression than patients on automatic PD and home HD45.
Faced with the problems identified in this transition, summarized in table IV, the main areas for improvement are:
1. Abolition of separate management models of dialysis modalities which promote care interruptions and untimely transitions among HD and PD.
2. Patient coaching in the transfer processes: anticipation and promotion of patient medical literacy and self‑determination during his therapy track33.
3. Preventive educational program for patients with PD to HD programmed transition, aimed at the HD autonomy of care and favouring the home‑integrated dialysis model41.
4. Revision of therapeutic targets, in order to integrate perception of quality of life[43], frailty, mental health, rehabilitation as already proposed in international groups36,37.
5. Evaluation of AVF creation in PD failure high‑risk patients40,41.
6. Promote, whenever possible, a planned transition between modalities, focusing on therapeutic adherence and patient well‑being and including cognitive, emotional and physical changes.
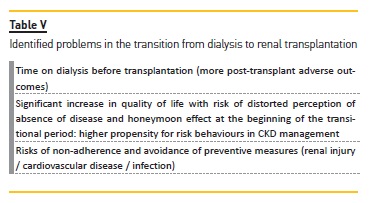
TRANSITION FROM DIALYSIS TO RENAL TRANSPLANTATION
Patients start to have a more positive perception of their disease when they shift from dialysis to renal transplantation27. According to Gill et al.47, during the transplantation process, the transition period from dialysis to transplantation is associated with an increased risk of death, with cardiovascular disease being the most common cause of death. This risk cannot be neglected but might be mitigated by adequate pre‑transplantation evaluation and risk stratification towards promptly adjusted protocols. The mortality rate is higher in patients who receive deceased donor kidney than those who receive living donor kidney47.
A longer dialysis period prior to transplantation is associated with higher mortality, psychological burden and likelihood of adverse outcomes (immunological or otherwise) after transplantation13,47. Observational studies with large samples suggest that, compared to patients on HD, those on PD may have a higher rate of graft thrombosis and early graft failure (at 3 months) after transplantation13. Patients on PD also appear to have shorter waiting time to transplantation, lower rate of delayed graft function and better long‑term graft survival13. However, differences in transplantation outcomes, according to previous dialysis modality, are probably more dependent on patient related variables (often insufficiently controlled for) than on the modality itself.
Von der Lippe et al48 evaluated the quality of life of patients transitioning from dialysis to renal transplantation.
Patients stated they had a better quality of life after transplantation, regardless of the modality of dialysis48. However, an improvement in quality of life was only sufficiently pronounced to be considered clinically relevant in specific kidney domains, namely renal disease burden, renal disease effects, symptoms and work situation, in addition to overall health48. This improvement was evident after more than three years post‑transplantation48. The quality of life results of renal transplant recipients were much lower than those of the general population, except in the domains of body pain and mental health48. These findings are plausible, since these are chronic patients who encounter challenges, such as short‑and long‑term risks of immunosuppression or deterioration of graft function, which contributes to the perception of a lower quality of life than the general population48.
According to another study49 into the impact of transitions between dialysis and transplantation on the quality of life and cognition of the disease, there was a clinically significant improvement in quality of life, with perception of fewer symptoms, consequences and presence of disease, in addition to a sensation of greater control over the disease between the period before and after transplantation. This improvement may reinforce a disease perception which is closer to an acute, instead of a chronic, event49. It should be noted that the very positive impact of transplantation may correspond to a honeymoon effect, which is known to occur in the initial period of this transition48,49.
Despite the positive results of this transition of care, some problems are identified (summarized in table V), so a comprehensive and coordinated multidisciplinary approach is still needed to promote a planned transition49 focused on therapeutic adherence. However, other measures of optimization are identified as major in this transition period:
1. Adoption of strategies to increase the number of kidney transplant recipients, in particular with the use of expanded criteria for kidney donors, destined for dialysis patients with a higher mortality risk, but having a survival advantage with transplantation47.
2. Adoption of strategies to improve mortality in wait‑listed patients (through prevention, diagnosis and treatment of cardiovascular disease) and in the peri‑transplant period (with reassessment of patients near the time of transplantation)47.
3. Promotion of organ donation.
TRANSITION BACK TO DIALYSIS AFTER RENAL TRANSPLANTATION
Advances in renal transplantation have led to more improvements in short‑term graft survival over long‑term survival, so that transplant failure with transition to dialysis will be inevitable with the end of graft survival35,49 or before, if complications develop.
One of the major threats of renal transplant patients is graft loss28. In the US graft failure is the fourth‑leading cause of dialysis initiation, following diabetes, hypertension and glomerulonephritis50.
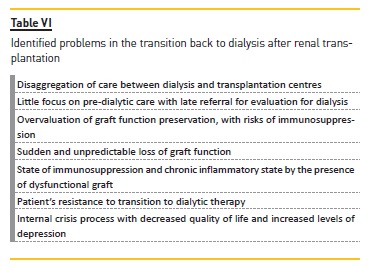
Transition from transplantation to dialysis should be more predictable and planned, but it can be a disorganized and discouraging process30. Patients with transplant failure may be late referenced for dialysis evaluation, even if they are followed by transplant nephrologists35. This may result from the disaggregation of care between dialysis and transplant centres, from the overvaluation of graft function preservation and little focus on pre‑dialytic care, delays induced by the patient or sudden and unpredictable loss of graft function35.
Patients with transplant failure have a higher risk of mortality than patients transitioning to advanced stage CKD49. According to Gill et al.47, the highest mortality rates are reached three months after graft loss, which corresponds to the period in which the cause of death due to sepsis is of greater importance. These authors also found a slightly higher mortality rate in patients who initiated HD after graft loss than those who started PD47. Mortality was also higher in patients with deceased donor than with live donor kidney47.
Graft failure is often reflected in decreased quality of life and increased levels of depression49. It is unequivocal that dialysis resumption is a crisis process for the failing renal graft recipient. A study35 that compared two groups of patients on dialysis found that patients with previous transplant failure had increased mortality, lower quality of life in its physical component, and a higher prevalence of diagnosed depression than non‑transplanted wait‑listed patients. Still in the group of patients with transplant failure, there was a greater probability of using CVC as HD access than AVF or graft, which is associated with an increased risk of death from infection35. These patients have lower serum albumin and haemoglobin values and higher PTH than never transplanted patients, although this difference is dissipated with time on dialysis35. The inferior results of these patients may be due to prolonged immunosuppression leading to known risks (neoplasms, infections, cardiovascular complications), or to the chronic inflammatory state associated with the presence of dysfunctional graft35. Another study49 which evaluated the impact of transitions between dialysis and transplantation on quality of life and illness cognitions, found a clinically significant decrease in quality of life, with perception of more symptoms, consequences and presence of renal disease, in addition to a feeling of less control over it, after transplant failure. It should be noted that transplantation programs response and re‑transplantations predicted time are highly conditioning variables on quality of life perception.
Faced with the problems identified in this transition, summarized in table VI, the main areas for improvement are:
1. Integration of multidisciplinary care in pre‑dialysis35,47 and reproduction of CKD management circuit in native kidneys.
2. Timely creation of arteriovenous vascular access or peritoneal access35,47.
3. Assessment of the most appropriate strategy for the patient and the readiness to reduce the dose of immunosuppressants after graft failure (to decrease sepsis deaths)35,47.
4. Evaluation of the possible adequacy of dysfunctional graft nephrectomy (to prevent chronic inflammatory state)35,47, when the patient meets clinical criteria.
5. Psychological support with patient coaching in crisis management and progression in self‑care in home dialysis programs.
TRANSITION TO END‑OF‑LIFE AND PALLIATIVE CARE
The aging of the population determines an increase in prevalence and incidence of chronic diseases, among which is CKD9. There is also an increase in the number of elderly patients undergoing dialysis or transplantation9.
The incidence of dialysis is increasing much more in the elderly over 75 years old than it is in patients in lower age groups51,52. RRT in patients over 75 years old is particularly associated with uncertainty about its effectiveness, given its poor results in advanced stage CKD9.
These patients have a slow decline, which is related to the presence of nephropathy with insignificant proteinuria51.
In the US, one‑year mortality of the elderly over 75 years of age who started dialysis is 41%51.
In a patient, the evolution pattern of eGFR and the presence of comorbidities are related to dialysis onset51. In the US, the elderly initiate dialysis relatively early, often in hospital setting and many of them in the absence of sufficiently specific or life‑threatening symptomatology51.
According to data from recent observational studies, dialysis does not seem to confer an advantage on survival, improvement of functional status or quality of life of the elderly with stage 5 CKD, functional limitation, much comorbidity or malnutrition51,53.
Verberne et al.54 compared survival between patients aged 70 years or over who chose RRT and CNT.
The average survival of those who chose RRT was higher than those who chose CNT54. However, the survival advantage associated with RRT was no longer seen in
patients aged 80 years or older and was very subtle in patients of 70 or more years with high comorbidity, which means that CNT may be a plausible alternative in selected patients54. Brown et al.55 compared the survival of patients who opted for CNT with those who chose RRT and also found no survival advantage in the RRT patient group over 75 years of age with two or more comorbidities. In the Foote et al.52 systematic review, no significant difference was found between the annual survival of PD or HD in the elderly compared to CNT, which suggests similar annual mortalities. However, these results may vary according to the comorbidities of the population treated, the type of access and the dialysis prescribers ability.
The majority of patients with advanced CKD is more favourable to care focused on reducing pain and suffering56.
It is necessary to review the therapeutic endpoints, dialysis quality assessment parameters and treatments in geriatric patients, already proposed internationally36,37.
According to the KDIGO Controversies Conference on Supportive Care in CKD57, comprehensive conservative care should be provided by a multidisciplinary team3,57 and is understood as holistic, patient‑centred care that includes interventions to delay the progression of kidney disease and minimize the risk of adverse events or complications; shared decision making; active symptom management; detailed communication including advanced care planning; psychological support; social and family support; cultural and spiritual domains of care57.
At this life stage, patients and clinicians still face institutional and structural insufficiencies in health services. There is a need for legal frameworks that support clinical decisions that do not always correspond to misplaced expectations of the family. Social support is also lacking.
The recognition of these limitations and the related economic pressure will be a first step towards obtaining new solutions. These will not only involve clinicians, but also social, institutional and community partners.
Faced with the problems encountered, summarized in table VII, the main areas for improvement in this transition are:
1. Assignment of a care coordinator to follow up the patient throughout the transition process and who provides the necessary information for an informed choice3,57.
2. Implementation of holistic conservative care programs targeted at selected patients for symptomatic control and focused on quality of life55,57.
3. Establishment of the legal framework that supports the application of these programs, giving the necessary support to clinical decisions.
4. Development of social support for patients in palliative care that counters the attitude of abandoning this group of patients in hospitals.
5. Gathering of social, institutional and community partners for the implementation of solution proposals that take into account the economic restraints inherent in this process.
CONCLUSION
The transitions in the course of CKD reviewed above are periods of great vulnerability, where critical decisions are made, and which have a high propensity for poor outcomes. This must be well‑known to be countered by anticipating the transition of care. Timely referral and nephrologist follow‑up are fundamental to reduce the difficulty of predicting the need to establish RRT. There is also an important role for the primary care physician in the patients clinical follow‑up and education.
The reality of CKD management is often a disaggregation of care, with deleterious consequences on patients follow‑up and quality of life. Investing in transition programs with multidisciplinary teams and coordination of care is fundamental to overcome this difficulty.
The choice of a centralizing healthcare practitioner, such as a nurse capable of managing the disease and to whom a patient would be assigned, would be a strategy to improve outcomes. This care centralizer would manage the disease requirements, establish a network of communication between different physicians, surveille the patient, promoting treatment compliance, educating for future transitions and avoiding duplication of unnecessary procedures. It is fundamental to transmit to patients a realistic perspective of the treatment trajectory, which should be focused on planning treatment transitions so that patients can make a process of preparation and change acceptance. This review aimed to emphasize the frequently documented deficiencies in the various transitional periods in CKD and to recognize its clinical, psychosocial and economic implications. The economic sustainability pressure of health services is the engine of better management solutions. The challenges are broad and the answers will not be immediate, but in each Unit, in each process, it is important to start, review, optimize and each step can mean the Change that is desired.
References
1. Jha V, Garcia‑Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives. Lancet Lond Engl 2013;382(9888):260–72. [ Links ]
2. Bello A, Alrukhaimi M, Ashuntantang G, Basnet S, C. Rotter R, G. Douthat W, et al. Complications of chronic kidney disease: current state, knowledge gaps, and strategy for action. Kidney Int Suppl 2017;7:122–9. [ Links ]
3. Johnson DS, Kapoian T, Taylor R, Meyer KB. Going Upstream: Coordination to Improve CKD Care. Semin Dial 2016;29(2):125–34. [ Links ]
4. Couchoud C, Couillerot A‑L, Dantony E, Elsensohn M‑H, Labeeuw M, Villar E, et al. Economic impact of a modification of the treatment trajectories of patients with end‑stage renal disease. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc – Eur Ren Assoc 2015;30(12):2054–68. [ Links ]
5. Sumida K, Kovesdy CP. Disease Trajectories Before ESRD: Implications for Clinical Management. Semin Nephrol 2017;37(2):132–43. [ Links ]
6. Rosansky SJ. Renal function trajectory is more important than chronic kidney disease stage for managing patients with chronic kidney disease. Am J Nephrol 2012;36(1):1–10. [ Links ]
7. Rastogi A, Linden A, Nissenson AR. Disease management in chronic kidney disease. Adv Chronic Kidney Dis 2008;15(1):19–28. [ Links ]
8. Sakhuja A, Hyland J, Simon JF. Managing advanced chronic kidney disease: a primary care guide. Cleve Clin J Med 2014;81(5):289–99. [ Links ]
9. Kalantar‑Zadeh K, Kovesdy CP, Streja E, Rhee CM, Soohoo M, Chen JLT, et al. Transition of care from pre‑dialysis prelude to renal replacement therapy: the blueprints of emerging research in advanced chronic kidney disease. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc – Eur Ren Assoc 2017;32(2):ii91‑ii98. [ Links ]
10. Levin A, Stevens PE. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int 2014;85(1):49–61. [ Links ]
11. Yee J. Improving Transitions in CKD: Failure Mode. Adv Chronic Kidney Dis 2016;23(4):211–4. [ Links ]
12. Lewicki MC, Polkinghorne KR, Kerr PG. Debate: Should dialysis at home be mandatory for all suitable ESRD patients?: home‑based dialysis therapies are the second choice after transplantation. Semin Dial 2015;28(2):147–54. [ Links ]
13. Fuquay R, Teitelbaum I. Transplant outcomes and dialysis modality. Contrib Nephrol 2012;178:251–7. [ Links ]
14. Couchoud C, Dantony E, Elsensohn M‑H, Villar E, Ecochard R, REIN Registry. Modelling treatment trajectories to optimize the organization of renal replacement therapy and public health decision‑making. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc – Eur Ren Assoc 2013;28(9):2372–82. [ Links ]
15. Sharief S, Hsu C. The Transition From the Pre‑ESRD to ESRD Phase of CKD: Much Remains to Be Learned. Am J Kidney Dis Off J Natl Kidney Found 2017;69(1):8–10. [ Links ]
16. Francis A, Johnson DW, Craig JC, Wong G. Moving on: transitioning young people with chronic kidney disease to adult care. Pediatr Nephrol 2018 Jun;33(6):973‑983. [ Links ]
17. Javalkar K, Fenton N, Cohen S, Ferris M. Socioecologic factors as predictors of readiness for self‑management and transition, medication adherence, and health care utilization among adolescents and young adults with chronic kidney disease. Prev Chronic Dis 2014;11:E117. [ Links ]
18. Bell LE, Ferris ME, Fenton N, Hooper SR. Health care transition for adolescents with CKD‑the journey from pediatric to adult care. Adv Chronic Kidney Dis 2011;18(5):384–90. [ Links ]
19. Ferris ME, Cuttance JR, Javalkar K, Cohen SE, Phillips A, Bickford K, et al. Self‑management and transition among adolescents/young adults with chronic or end‑stage kidney disease. Blood Purif 2015;39(1–3):99–104. [ Links ]
20. Foster BJ. Heightened graft failure risk during emerging adulthood and transition to adult care. Pediatr Nephrol Berl Ger 2015;30(4):567–76. [ Links ]
21. Ferris ME, Mahan JD. Pediatric chronic kidney disease and the process of health care transition. Semin Nephrol 2009;29(4):435–44. [ Links ]
22. Akchurin OM, Melamed ML, Hashim BL, Kaskel FJ, Del Rio M. Medication adherence in the transition of adolescent kidney transplant recipients to the adult care. Pediatr Transplant 2014;18(5):538–48. [ Links ]
23. Watson AR, Harden PN, Ferris ME, Kerr PG, Mahan JD, Ramzy MF, et al. Transition from pediatric to adult renal services: a consensus statement by the International Society of Nephrology (ISN) and the International Pediatric Nephrology Association (IPNA). Kidney Int 2011;80(7):704–7. [ Links ]
24. Alpay H. Transition of the adolescent patient to the adult clinic. Perit Dial Int J Int Soc Perit Dial 2009;29(2):S180–2. [ Links ]
25. Kaspar CDW, Bholah R, Bunchman TE. A Review of Pediatric Chronic Kidney Disease. Blood Purif 2016;41(1–3):211–7. [ Links ]
26. Samuel SM, Nettel‑Aguirre A, Soo A, Hemmelgarn B, Tonelli M, Foster B. Avoidable hospitalizations in youth with kidney failure after transfer to or with only adult care. Pediatrics 2014;133(4):e993–1000. [ Links ]
27. Sébille V, Hardouin J‑B, Giral M, Bonnaud‑Antignac A, Tessier P, Papuchon E, et al. Prospective, multicenter, controlled study of quality of life, psychological adjustment process and medical outcomes of patients receiving a preemptive kidney transplant compared to a similar population of recipients after a dialysis period of less than three years–The PreKit‑QoL study protocol. BMC Nephrol 2016;17:11. [ Links ]
28. von der Lippe N, Waldum B, Østhus T‑BH, Reisæter AV, Os I. Health related quality of life in patients in dialysis after renal graft loss and effect of gender. BMC Womens Health 2014;14(1):34. [ Links ]
29. Fluck R. Transitions in care: what is the role of peritoneal dialysis? Perit Dial Int J Int Soc Perit Dial 2008;28(6):591–5. [ Links ]
30. Blake PG. Difficult «transitions» for renal patients. Perit Dial Int J Int Soc Perit Dial 2008;28(6):575. [ Links ]
31. Nessim SJ, Bargman JM, Jassal SV, Oliver MJ, Na Y, Perl J. The impact of transfer from hemodialysis on peritoneal dialysis technique survival. Perit Dial Int J Int Soc Perit Dial 2015;35(3):297–305. [ Links ]
32. Zhang A‑H, Bargman JM, Lok CE, Porter E, Mendez M, Oreopoulos DG, et al. Dialysis modality choices among chronic kidney disease patients: identifying the gaps to support patients on home‑based therapies. Int Urol Nephrol 2010;42(3):759–64. [ Links ]
33. Hutchinson TA. Transitions in the lives of patients with End Stage Renal Disease: a cause of suffering and an opportunity for healing. Palliat Med 2005;19(4):270–7. [ Links ]
34. Hanko J, Jastrzebski J, Nieva C, White L, Li G, Zalunardo N. Dedication of a nurse to educating suboptimal haemodialysis starts improved transition to independent modalities of renal replacement therapy. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc – Eur Ren Assoc 2011;26(7):2302–8. [ Links ]
35. Perl J, Zhang J, Gillespie B, Wikström B, Fort J, Hasegawa T, et al. Reduced survival and quality of life following return to dialysis after transplant failure: the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc – Eur Ren Assoc 2012;27(12):4464–72. [ Links ]
36. Chronic Kidney Disease | ICHOM – International Consortium for Health Outcomes Measurement (Internet). Disponível em: http://www.ichom.org/medical‑conditions/chronic‑kidney‑disease/. Consultado pela última vez a 2018/05/17. [ Links ]
37. Older Person | ICHOM – International Consortium for Health Outcomes Measurement (Internet). Disponível em: http://www.ichom.org/medical‑conditions/older‑person/. Consultado pela última vez a 2018/05/17. [ Links ]
38. Lukowsky LR, Mehrotra R, Kheifets L, Arah OA, Nissenson AR, Kalantar‑Zadeh K. Comparing Mortality of Peritoneal and Hemodialysis Patients in the First 2 Years of Dialysis Therapy: A Marginal Structural Model Analysis. Clin J Am Soc Nephrol CJASN 2013;8(4):619–28. [ Links ]
39. Pajek J, Hutchison AJ, Bhutani S, Brenchley PEC, Hurst H, Perme MP, et al. Outcomes of peritoneal dialysis patients and switching to hemodialysis: a competing risks analysis. Perit Dial Int J Int Soc Perit Dial 2014;34(3):289–98. [ Links ]
40. Chiarelli G, Beaulieu M, Cozzolino M, Singh S, Kiaii M, Taylor P, et al. Vascular access planning in peritoneal dialysis patients. Perit Dial Int J Int Soc Perit Dial 2008;28(6):585–90. [ Links ]
41. Boissinot L, Landru I, Cardineau E, Zagdoun E, Ryckelycnk J‑P, Lobbedez T. Is transition between peritoneal dialysis and hemodialysis really a gradual process? Perit Dial Int J Int Soc Perit Dial 2013;33(4):391–7. [ Links ]
42. Nadeau‑Fredette A‑C, Bargman JM, Chan CT. Clinical outcome of home hemodialysis in patients with previous peritoneal dialysis exposure: evaluation of the integrated home dialysis model. Perit Dial Int J Int Soc Perit Dial 2015;35(3):316–23. [ Links ]
43. Shrestha S, Ghotekar LR, Sharma SK, Shangwa PM, Karki P. Assessment of quality of life in patients of end stage renal disease on different modalities of treatment. JNMA J Nepal Med Assoc 2008;47(169):1–6. [ Links ]
44. United States Renal Data System. 2017 USRDS annual data report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2017. [ Links ]
45. Griva K, Davenport A, Harrison M, Newman S. An evaluation of illness, treatment perceptions, and depression in hospital ‑ vs. home‑based dialysis modalities. J Psychosom Res 2010;69(4):363–70. [ Links ]
46. Ipema KJR, van der Schans CP, Vonk N, de Vries JM, Westerhuis R, Duym E, et al. A difference between day and night: protein intake improves after the transition from conventional to frequent nocturnal home hemodialysis. J Ren Nutr Off J Counc Ren Nutr Natl Kidney Found 2012;22(3):365–72. [ Links ]
47. Gill JS, Rose C, Pereira BJG, Tonelli M. The importance of transitions between dialysis and transplantation in the care of end‑stage renal disease patients. Kidney Int 2007;71(5):442–7. [ Links ]
48. von der Lippe N, Waldum B, Brekke FB, Amro AAG, Reisæter AV, Os I. From dialysis to transplantation: a 5‑year longitudinal study on self‑reported quality of life. BMC Nephrol 2014;15:191. [ Links ]
49. Griva K, Davenport A, Harrison M, Newman SP. The impact of treatment transitions between dialysis and transplantation on illness cognitions and quality of life – a prospective study. Br J Health Psychol 2012;17(4):812–27. [ Links ]
50. Mehrotra A, Tan JA, Ames SA. «Out of Sight, Out of Mind»: The Failed Renal Allograft as a Cause of ESA Resistance. Semin Dial 2015;28(5):530–2. [ Links ]
51. Rosansky SJ, Schell J, Shega J, Scherer J, Jacobs L, Couchoud C, et al. Treatment decisions for older adults with advanced chronic kidney disease. BMC Nephrol 2017;18(1):200. [ Links ]
52. Foote C, Kotwal S, Gallagher M, Cass A, Brown M, Jardine M. Survival outcomes of supportive care versus dialysis therapies for elderly patients with end‑stage
Kidney disease: A systematic review and meta‑analysis. Nephrol Carlton Vic 2016;21(3):241–53.
53. Tam‑Tham H, Thomas CM. Does the Evidence Support Conservative Management as an Alternative to Dialysis for Older Patients with Advanced Kidney Disease? Clin J Am Soc Nephrol CJASN 2016;11(4):552–4. [ Links ]
54. Verberne WR, Geers ABMT, Jellema WT, Vincent HH, van Delden JJM, Bos WJW. Comparative Survival among Older Adults with Advanced Kidney Disease Managed Conservatively Versus with Dialysis. Clin J Am Soc Nephrol CJASN 2016;11(4):633–40. [ Links ]
55. Brown MA, Collett GK, Josland EA, Foote C, Li Q, Brennan FP. CKD in elderly patients managed without dialysis: survival, symptoms, and quality of life. Clin J Am Soc Nephrol CJASN 2015;10(2):260–8. [ Links ]
56. Grubbs V, Moss AH, Cohen LM, Fischer MJ, Germain MJ, Jassal SV, et al. A palliative approach to dialysis care: a patient‑centered transition to the end of life. Clin J Am Soc Nephrol CJASN 2014;9(12):2203–9. [ Links ]
57. Davison SN, Levin A, Moss AH, Jha V, Brown EA, Brennan F, et al. Executive summary of the KDIGO Controversies Conference on Supportive Care in Chronic Kidney Disease: developing a roadmap to improving quality care. Kidney Int 2015;88(3):447–59. [ Links ]
Isabel Marques Correia, MD
E‑mail: isabelmmcorreia@gmail.com
Disclosure of potential conflicts of interest: none declared.
Received for publication: Jul 9, 2018
Accepted in revised form: Sep 10, 2018














