Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Portuguese Journal of Nephrology & Hypertension
Print version ISSN 0872-0169
Port J Nephrol Hypert vol.32 no.2 Lisboa June 2018
REVIEW ARTICLE
Supportive care in advanced chronic kidney disease: Withholding and withdrawing dialysis therapy
C Belino1, C Meng2, R Neto2,3, E Gonçalves4
1 Department of Nephrology, Centro Hospitalar de Vila Nova de Gaia e Espinho, Vila Nova de Gaia, Portugal
2 Department of Nephrology, Hospital de São João, Porto, Portugal
3 Department of Nephrology, Pre-dialysis Consult, Hospital de São João, Porto, Portugal
4 Department of Pallitave Care, Hospital de São João, Porto, Portugal
ABSTRACT
Over the latest few decades, dialysis has been offered to older and more complex patients. This treatment can increase the symptom burden and also add new symptoms that can have a profound impact in frail and/or elderly patients with multiple comorbidities. A quality of life approach may be more desirable than a quantity of life approach in these cases. Around the world, some countries have endorsed programs of shared decisionmaking process and advanced care planning for end-stage renal disease, with creation of goal-directed protocols.
Alignment with palliative care programs to develop structured approaches is the key to successful outcomes.
Reforms in medical education are needed to address current necessities in these areas. This article summarizes current knowledge regarding decision making and palliative care in end-stage renal disease.
Keywords: dialysis withdraw, time-limited trial, renal palliative care
INTRODUCTION
The prevalence of end-stage kidney disease continues to increase, along with life expectancy. International data shows a high mortality in elderly patients initiating dialysis therapy and the majority had severe chronic illnesses1-4. These patients will probably benefit more from an integrated individual approach prioritizing quality of life instead of a disease approach of prolonging life, frequently with more hospice use and interference with social and family spheres. Adequate shared decision-making process and advanced care planning with proper assessment and management of symptoms are fundamental. In this article, we will discuss the principles and issues related to decisions to forgo dialysis and palliative care of ESRD patients.
ETHICAL PRINCIPLES REGARDING
DECISIONS TO FORGO DIALYSIS
The ethical principles of beneficence, non-maleficence, autonomy, justice and professional integrity must undergo all clinical decisions. Considering these principles, the Renal Physicians Association (RPA) published a group of practice guidelines1 that identified some conditions in which is ethical to withhold or withdraw dialysis, many of which were further incorporated in the KDIGO 2015 conference2 on supportive care in CKD:
1. Patients with decision-making capacity who, being fully informed and making voluntary choices refuse dialysis or request discontinuation of therapy.
2. Patients who no longer possess decision-making capacity who have previously indicated refusal of dialysis therapy in an advance oral or written directive.
3. Patients who no longer possess decision-making capacity and whose properly appointed legal agents/surrogates refuse dialysis therapy or request to be discontinued.
4. Patients with irreversible profound neurologic impairment such that they lack signs of though, sensation, purposeful behavior, and awareness of self and environment.
5. It is reasonable to consider not initiating or withdrawing dialysis for patients with acute renal failure or ESRD who have a terminal illness (life expectancy ≤ 6 months) from a nonrenal cause or whose medical condition precludes the technical process of dialysis.
Specifically, nephrologists can only provide treatments that offer reasonable expectation of benefit without unacceptable harm and center them on patient autonomy, implying that the patient (or his legal substitute) is the best person to make his own health care decisions. Dialysis should only be provided if it meets individual goals and if it doesn´t, care should focus on treating symptoms and quality of life. For those requiring dialysis with an uncertain prognosis or for whom a consensus cannot be reached about providing dialysis, nephrologist should consider offering a time-limited trial of dialysis1-5. Regarding withdrawal from dialysis, KDIGO states that this is ethically and clinically acceptable after a process of shared decision making but before this, all potentially remedial factors contributing to this decision such as depression, pain or other symptoms, should be addressed as well as the potentially reversible social factors2.
CULTURAL, ETHNIC AND RELIGIOUS CONCERNS
End-of-life care preferences can vary according ethnicity, cultural practices and religious beliefs1,2,5. A family-centered model of decision making may be preferred and some patients may desire that their community receive and disclose information before a decision is made, even when the patient is competent.
Alternatively, resistance to forgo dialysis despite reduced benefit may reflect patient´s need to extend life to fulfill moral duties. In some religions, not starting dialysis may represent a lack of faith in divine intervention.
For this, it may be difficult to discuss illness course and prognosis in some cases4,5. Nephrologist and other health care professionals will need to determine how the patient wishes to receive and discuss information and make decisions. Discussions and decisions should occur in a culturally appropriate context and with a cultural appropriate decision-making team2.
LEGAL ASPECTS
Competent patients have the right to consent to or decline a medical treatment. The decision should only be made after a full explanation of diagnosis, prognosis and all treatment options to each patient. According to RPA guidelines1, explanation of treatment options should include: (1) available dialysis modalities; (2) not starting dialysis and continuing conservative management which should include end-of-life care; (3) a timelimited trial of dialysis; and (4) stopping dialysis and receiving end-of-life care. Final decision should be informed and voluntary and the medical team must ensure that the patient or his legal agent understand the consequences of the decision. In several countries, an official document with expression of informed consent or refusal must be signed by the patient or his legal agent1,4. In Portugal, the Directorate-General for Health (in Portuguese, Direção-Geral da Saúde) norm 017/2011 includes the Portuguese version of this document that must be signed by all patients with advanced kidney disease, after being properly informed.
Informed consent is a process prescribed by law which has seven elements6:
Threshold elements (preconditions): decisionmaking capacity, voluntariness
Information elements: disclosure of material information, recommendation of a plan, understanding the information and recommendation
Consent elements: decision in favor a plan, authorization of a plan.
Currently, there is concerning discrepancy between legal and current practice6. A US study which evaluated older patients on hemodialysis revealed that most of them lacked sufficient understanding of their clinical circumstances7. Also, in a group of observation studies, only a minority reported that dialysis initiation was their choice8. In a Canadian study9, 61% of patients regretted commencing dialysis and 52% of them reported that it was their physicians wish and 14% said that is was their family´s wish. Clarifying this issue is of crucial importance to avoid suffer and waste of health resources.
If the nephrologist is not sure of a patient´s capacity to make informed consent, this should be confirmed with a formal assessment or referral6.
PRACTICE PATTERNS
There is a lack of evidence regarding the patterns and frequency of withholding dialysis therapy. The DOPPS (Dialysis Outcomes and Practice Patterns Study) showed a great variance in nephrologist´s practices10.
According to the Dialysis and Transplant Registration of the Spanish Society of Nephrology, about 60% of the patients with CKD stage 5 do not receive renal replacement therapy due to one of the following reasons: death, lack of clinical suitability for dialysis or the unawareness of the disease. In different Spanish studies, prevalence of CKD stage 5 patients on conservative treatment varied between 8 to 65%, with a mean of 39%11.
Recently, in a large USA survey of views and practices patterns of dialysis medical directors towards end-of-life decision-making in ESRD, the majority of respondents felt very prepared (66%) or somewhat prepared (29%) and most (80%) endorsed a model of shared decision making. If asked to do so, 70% of the respondents provided prognostic information often or nearly always. For patients with a poor prognosis, 36% of responders would offer a time limited trial of dialysis and 56% would recommend withdrawal from dialysis if patients were already receiving this therapy12. A similar survey made in Europe by the European Renal Association – European Dialysis and Transplant Association (ERA-EDTA) showed a different reality13. About 42% reported occurrence of withdrawal in their dialysis unit and 56% perceived life-prolonging treatments in terminally ill patients was allowed. Only 7% of the responders reported the presence of protocols in their units on withdrawal decision making (7%) or palliative care (10%) or the common involvement of a geriatrician in withdrawal decisions (10%). The majority reported that palliative care had not been part of their core curriculum (74%) and had not attended medical education sessions on this topic. Occurrence reports were more likely in respondents worked in a public center, if stopping life-prolonging therapy was perceived as allowed, if withdrawal decisions were considered shared between doctors and patients and if reimbursement of palliative care was believed to be in place.
Efforts are necessary to educate properly the nephrologists regarding the shared–decision-making process and end-of-life decisions and care in order to change this reality. British nephrologists already have formal programs for care of patients who have chosen to withhold or withdraw from dialysis. Patients in these conservative care programs receive usual integrated CKD care allied with palliative or supported care as well4,14.
TIME-LIMITED TRIAL OF DIALYSIS
Time-limited trials (TLT) can be defined as An agreement between clinicians and patient/surrogate decisionmakers to use medical therapies such as mechanical ventilation, enteral feeding, or dialysis over a defined period of time to determine if the patient improves or deteriorates according to agreed-upon clinical outcomes15.
A TLT of dialysis has been considered an acceptable option when there is doubt if the patient will benefit from dialysis, if the patient´s prognosis or response to treatment is uncertain and persistence with burdensome therapies seems undesirable and when a lack of consensus among the medical team and family exists1,15-17.
There are other additional advantages such as alleviating some of the burden experienced by families when asked to choose a treatment in face of uncertainty or offering an opportunity for forecasting a poor prognosis, giving families time to emotionally prepare before the death of a love one and helping to avoid professional conflicts between the medical team and patient/family17. However, a TLT is appropriate only when there is a reasonable chance that dialysis therapy will have a net benefit for patient and that patient´s goals are achieved4.
When a trial is implemented, it is necessary to establish clear parameters and timelines in order to determine at the end of the trial if dialysis therapy should be continued or not. Quill and Holloway15 proposed a five-step framework for the management of a timelimited trial (Table 1). To be effective, a written contract between both the physician and the patient/legal agent should be drawn up and signed. In this document should list the patient´s current clinical condition and the duration of TLT with clear goals that must be met for dialysis to be continued. If the goals are not reached, dialysis is discontinued and aggressive palliative care is provided4,16. This document, despite the difficulty of achievement in clinical practice, helps to overcome possible conflicts between dysfunctional families with non-adherence to TLT or families who want everything done and the nephrologist and when there is no consensus in medical team15,16.
When to start and when to stop the Trial
Deciding when to begin the trial is a complex and difficult decision. Although previously there was a trend to start dialysis early (eGFR> 10 ml/min/1.73 m2), evidence from recent years including the IDEAL (Initiating Dialysis Early and Late) trial showed no benefit of an early start4,18. Rosansky et al18 suggested that the severity of CKD may be overdiagnosed in elderly or very ill patients based on inaccuracies in eGFR estimation and this may result in unnecessary dialysis initiation. O´Hare et al19, using data from the Veterans Administration population, reported that elderly have slower progression of CKD and most are more likely to die than progress to end-stage kidney disease (ESRD). This mortality competing risk becomes more notable with ageing, with <1% of elderly with CKD progressing to a final state and subsequent dialysis each year4. Some authors have suggested that elderly patients with CKD will benefit more from and individualized approach with maximization of quality of live instead the traditional disease approach2-4.
Moreover, when forthcoming the decision to start dialytic therapy and TLT, some aspects should be provided and pondered before reaching a decision (Table 2).
Regular symptom assessment using scales and prognostic tools may help to estimate trajectory illness and find the point in which the benefit of dialysis will overcome the risks2. Adhesion to medical regime is also essential for proper management2-4.
The usual duration of a TLT in context of acute deterioration of renal function is few days to 2 weeks and 1-3 months for ESRD. Specific pause point for clinical re-evaluation are important to check for accomplishment of goals and identification of burdens and sentinel events that may indicate more benefit to specialized services such palliative care, with an eventually early termination of trial. If at the completion of the first trial the treatment outcomes remain uncertain and/or if doubt is regarding the achievement of patient´s goals, is licit to perform another trial16. Unfortunately, there is no information about the frequency or outcomes of TLT.
Choice of dialysis modality
Patients who reach TLT are usually older and/or with greater number of comorbidities, risk of cognitive dysfunction and higher levels of frailty4,20. There are few studies who addressed dialysis outcomes in this group of patients. Given the social burden of and propensity for functional limitations, the self-care dialysis treatment options are most limited20. In the United States, only 2.5% of patients aged >65 years are on PD21. The numbers are better in Europe, about 10 – 15%20,21.
HD is challenging for these patients especially because of hypotension and negative impact on myocardial and cerebral functioning, risks of increasing inflammatory markers, the complexity of creating and maintaining a vascular access, post-dialysis recovery time and the risk of falls after dialysis20. Transportation issues may interfere with social and family life. However, there is also a social structure related to dialysis unit, with regular medical review when attending treatments and the procedure is done by others. Other advantages of HD are related with efficient solute and volume removal particularly in anuric patients, limited time spent of dialysis and freedom for the patient and his family form involvement with dialysis procedure itself20.
Otherwise, the main advantage of PD is the possibility of home-based therapies, with flexibility of treatments especially if there is some RRF left and avoidance of multiple visits to hospital or clinic regardless of whether and how the patient is feeling20. Assisted PD can overcome functional and sensorial impairment barriers.
With planning and appropriate information there is no evidence of PD-related complications being more common in these patients20. PD seems to confer less risk for dementia, hemorrhagic stroke and subdural hematomas, despite equivalent risk of falls. Outcomes of survival and quality of life are similar between two techniques at least at 6 and 12 months20,22,23.
Considering this, the optimal modality for TLT is individualized according to patient and family characteristic and wishes.
Dialysis dosage
The amount of dialysis that should be prescribed for patients on TLT has not yet been defined. The updated KDOQI guidelines24 generally suggest a minimal target of Kt/V urea of 1.2 per HD treatment given 3 times per week. More frequent treatments (5-7 per week) have been shown to reduce recovery time, respiratory distress and sleep disorders and to improve cardiovascular function and quality of life but in a long-term way (12 months)25. In short-term, more frequent treatments may enable better rehabilitation. However, treatment burden and access issues must be balanced20. For patients with RRF, a customized approach with shorter periods of treatment time or only 2 times per week as part of an incremental hemodialysis regime has been shown to improve results20. Calculation of PD clearance already includes RRF, enabling also an incremental increase in PD prescription as renal function declines, besides flexibility of treatment (automatic versus continuous ambulatory PD)20.
Like the modality chosen, the optimal dialytic regimen for TLT is individualized and upgraded according to patient characteristics and results.
THE CONCEPT OF PALLIATIVE DIALYSIS
A palliative approach to dialysis can be defined as a transition from a disease-oriented focus on dialysis as rehabilitative treatment to an approach prioritizing comfort and alignment with patients preferences and goals to improve quality of life and reduce symptom burden for maintenance dialysis patients in their final year of life. This transition aligns generally with palliative care. Table 3 summarizes the major sentinel events signalizing this period26-31.
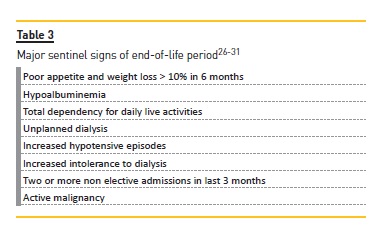
A central venous catheter can be acceptable, as also lower clearances if changes in dialysis prescription increase demands inconsistent with patient preference. Hypertension can be tolerated to avoid symptoms, no indication for dyslipidemia treatment. Reduction of dietary restrictions (with more permissive hyperphosphatemia) can have a major impact in quality of life. Laboratory monitoring should be the minimal necessary26.
It is important to note that palliative dialysis is not equivalent to less dialysis or a precursor to withdrawal of dialysis because this alone will not reduce symptoms or suffering of patients. Less dialysis rarely provides benefits and can aggravate symptoms and post-dialysis fatigue, especially if greater ultrafiltration is needed. Also, alterations in dosing and timing of dialysis session can affect the patient. Per example, stress associated with rushing for an earlier or later dialysis session or change in eating patterns when attending dialysis. Engaging families in frequent discussions will help to identify conditions that can be optimized. Even minor issues like small changes in dialysis schedule or location may improve wellbeing and dialysis tolerance27.
PALLIATIVE/SUPPORTIVE CARE
Palliative care should be offered to all patients with ESRD, despite decision to withhold or withdraw from dialysis. The evolving concept of palliative care is the providence of support through the course of a person´s chronic disease rather just at the end of life.
Symptom burden and management in ESRD
The symptom burden is similar to that of patients with terminal heart failure or cancer28. Figure 1 presents the prevalence of major symptoms in renal disease28.
Figure 2 resumes the non-pharmacologic and pharmacologic approaches to major symptoms (made by the authors).
Fatigue is frequent and disabling in ESRD patients. Usually is described as a complex and poor defined constellation of symptoms like physical sleepiness, lack of energy, lethargy and weakness, being closely related with depressive symptoms. Causes that may contribute to fatigue are anemia, inadequate dialysis, post-dialysis fatigue and efforts associated with attending dialysis sessions or hospital visits. Other modifiable contributing factors are vitamin D deficiency, metabolic acidosis, tertiary hyperparathyroidism, hypothyroid, mood disorders; sleep disorders, malnutrition and polypharmacy(26-29). Evidence regarding treatment is driven from palliative studies in other settings, since ESRD patients are usually excluded28. Evidence from cancer literature suggests a benefit with use of methylphenidate 5mg per day (can be increased up to 20 mg per day)30. However, adverse effects related with appetite reduction may increase malnutrition and lead to further frailty.
Some authors31 recommend fluoxetine 20 mg or sertraline 50 mg orally per day. More safe approaches are psychotherapy, correction of modifiable factors, reduction of post-dialysis fatigue (e.g. increased frequency) and increase resistance and muscle strength with lowintensity resistance and aerobic exercise. Fatigue usually occurs in a complex cluster with other symptoms and correction of sleep and mood disorder can improve significantly the subjective sensation of fatigue28.
Pruritus and itchy skin are also one of the most bothersome symptoms, with significant impact in quality of life and related with poor sleep and depression. The causes are multifactorial: anemia, iron deficiency, hypercalcemia, hyperphosphatemia and other uremic toxins, xerosis, allergies, drug sensitivities and contact dermatitis. The highest levels of evidence for efficacy are for topical agents, oral medications and ultraviolet B therapy. Topical emollients are first-line therapies; they should be water-based and be fragrance and additives free. They should be applied 2-3 times daily.
Agents that help to cool skin such as a fan or topical camphor/menthol, especially at night, also have good results. Other topical therapies such as gamma-linolenic acid 2.2% cream applied twice daily, capsaicin 0.025% or 0.03% applied 2-4 times daily may be valuable adjutants.
Oral medications should be considered if the above is not effective and pruritus is affecting quality of life. Low-dose gabapentin starting at 50-100 mg postdialysis or second-line doxepin 10 mg nightly are good options. Mirtazapine reduces central sensitization to itch and is also an option, starting dose of 15 mg daily.
Antihistamines do not reduce uremic pruritus; however, the sedative effect may help with sleep disturbance.
There is some evidence for ondasentron 4 mg orally every 8 hours or naloxone 50 mg orally per day31. Other therapies with less evidence include UVB phototherapy 3 times per week and acupuncture2,27,28.
Breathlessness/shortness of breath is very distressful for ESRD patients. Major causes are anemia, hypervolemia with pulmonary edema and metabolic acidosis.
Correction of these modifiable factors with medical therapy (erythropoiesis-stimulating agents, diuretics and sodium bicarbonate) or adjustment in dialysis prescription with ultrafiltration intensification may be needed. Encouragement of physical activity in selected cases might be helpful2,27,28. If anxiety is a significant component, low-dose benzodiazepines such as lorazepam or diazepam may be helpful. Domiciliary oxygen may be needed, especially in cases of advanced pulmonary disease31. Low dose opioids can also be given, but should be chosen carefully and monitored to avoid toxicity28.
Sleep disorders are common and mostly secondary to restless leg syndrome (RLS), pruritus, pain, dyspnea, mood disorders, obstructive sleep apnea and certain medications. Non-pharmacologic treatment should be considered first, like relaxation therapy or cognitive behavioral therapies, promotion of sleep hygiene (avoid napping during the day, reduce stimulants such as caffeine, alcohol and nicotine). If those are unsuccessful, consider low-dose gabapentin post-dialysis, melatonin, zolpidem 5-10 mg nightly, doxepin 10 mg nightly or temazepam 15 mg orally at bedtime28,31. Management of secondary causes is fundamental.
Restless leg syndrome (RLS) is particularly common in dialysis patients, reaching a prevalence of 10-20%.
About 80% of affected patients also have the sleep disorder periodic limb movements. Besides reducing quality of life, RLS is also associated with increased cardiovascular morbidity and mortality. Specific cause is unknown2,27-32. Modifiable contributing factors have been identified: anemia, iron deficiency, hyperphosphatemia and medications such as dopamine antagonists, serotonin–norepinephrine reuptake inhibitors, tricyclic antidepressants, calcium channel blockers, opioids. Consider nonpharmacologic therapy first, like intradialytic aerobic exercise, removal of stimulants and dopamine antagonists, good sleep hygiene, pneumatic compression devices and correction of modifiable factors.
If unsuccessful, low-dose gabapentin or pregabalin (25 mg) after dialysis may help and second-line options include dopamine agonists such as levodopa 50-200 mg nightly, ropinirole 0.25-3 mg/day and pramipexole 0.125-0.5 mg nightly. Clonazepam 0.5-2 mg/day nightly can also improve symptoms, mainly because of a sedative effect27-31.
Refractory Cramps, also common in these patients, can be managed with quinine sulphate 200-300 mg nightly31.
Depressive symptoms occur frequently along the entire spectrum of CKD. Lifetime risk of depression in these patients is about 39% compared with 7% in general population27. Depression augments the risk of hospitalization, mortality and withdrawal from dialysis.
Usually is associated with other symptoms like pain, poor sleep and pruritus that have to be managed. More frequent dialysis, cognitive behavioral therapy and exercise programs are the major nonpharmacological options. Antidepressants like fluoxetine 20-40 mg, sertraline 50-100 mg, paroxetine10-40 mg, escitalopram 10-20 mg daily can be effective drugs in these patients27,31. Tricyclic antidepressants are usually poorly tolerated and abuse of benzodiazepines increases mortality risk.
Gastrointestinal symptoms like anorexia, nausea, vomiting, constipation and diarrhea are also frequent in ESRD patients. Uremia is a powerful nausea inductor and contributes to gastrointestinal hypomotility, which can be aggravated by diabetes mellitus. Many drugs used commonly in CKD like phosphate binders, iron, vitamin D analogues, antibiotics or antidepressants can induce gastrointestinal intolerance. Good oral hygiene and smaller but frequent meals cooked simply without excessive grease, spice and sweetness can improve symptoms. Applying a cool, damp cloth to forehead or nape of neck and loose-fitting clothing can also help.
Useful drugs are metoclopramide 2.5 mg PO/SC 4/4hours, domperidone orally 10 mg 2-3 times daily if intolerance to metoclopramide, ondansetron 4 mg orally 8/8 hours, haloperidol 0.5 mg PO/SC 4/4 hours, olanzapine 2.5 mg PO 4/4hours. If usual antiemetics are ineffective, levopromazine 6 mg PO/SC once daily may be tried27-31.
Anorexia has been associated with malnutrition, weight loss, fatigue and falls, with greater hospitalization rates and mortality. Adequate dialysis should be ensured, dry mouth (salivix pastilles) and gastroparesia treatment should be managed. Consider mirtazapine 15 – 50 mg/day, dronabinol 2.5 mg orally before meals, megestrol 400 mg or prednisolone 10 mg orally per day29-32.
Pain is well documented in more than 50% of CKD patients. There are five types of pain: renal-specific pain (polycystic kidneys, amyloid, and calciphylaxis), dialysisspecific pain (steel syndrome, headache, fistula problems, and abdominal pain from PD), musculoskeletal pain (renal osteodystrophy, muscle spasms and cramps, carpal tunnel syndrome), neuropathic pain (renal or diabetic neuropathy) and ischemic pain (peripheral vascular disease, vasculitis). Exercise, cognitive and psychological approaches can minorate pain sensation. A step-wise approach to analgesics such as outlined in World Health Organization (WHO) Analgesic Ladder is recommended. Analgesic selection, initial dosing and titration must be individualized and according to type of pain27,32 (Figure 3 – proposed by the authors, based on WHO Analgesic Ladder and Gloucestershire NHS Foundation Hospitals – Guidelines for End of Life Care in Advanced Kidney Disease). Before starting chronic opioid therapy (moderate to severe pain), risks of substance abuse, misuse or addiction should be addressed.
Morphine, codeine, meperidine and propoxyphene have neurotoxic metabolites that are excreted by the kidneys and that accumulate in CKD with a high likelihood of toxicity, and are not recommend as a first line27-32.
Last days of life
Patients should be given the choice to die at home with hospice care or wherever they prefer, if there is sufficient and appropriate support to enable this option. When a patient decides to withdraw from dialysis, it is important to prepare him and his family that survival average is 7.4 days (range 0 – 40). A specialist in palliative care is fundamental in this process. Agitation and confusion are best managed using a combination of haloperidol and a benzodiazepine.
Pain is better addressed with intravenous or subcutaneous opioids. Dyspnea can be relived with oxygen, bronchodilators and a combination of low dose opioids and short-acting benzodiazepines like midazolam to decrease respiratory effort. Respiratory tract secretions can be reduced with use of hyoscine butylbromide and glycopyrronium. Haloperidol can be used to treat myoclonus, nausea and vomiting, ondansetron is also effective for nausea and uremic pruritus. Bereavement support should also be offered to patients families2,4,27-32.
CONCLUSIONS
The scenario of withholding and withdrawing dialysis will become increasingly more frequent in the near future, since the ESRD patient is now typically elderly with multiple comorbidities and life expectancy is increasingly growing. Nephrologists are poorly prepared to deal with end of life decisions and to engage in shared decision making and advanced care planning. Efforts in medical education and creation of specialized programs and protocols with palliative care team are essential towards a better medical care and fulfillment of patient, family and also the doctors goals and perspectives.
References
1. Renal Physicians Association. Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis. 2nd ed. Rockville, MD: Renal Physicians Association; 2010. [ Links ]
2. Davison SN, Levin A, Moss AH, Jha V, Brown EA, Brenann F, et al. Executive Summary of the KDIGO Controversies Conference on Supportive Care in Chronic Kidney Disease: developing a roadmap to improving quality care. Kidney Int. 2015;88(3):447-59. [ Links ]
3. Holley JL. We offer renal replacement therapy to patients who are not benefitted by it. Semin Dial. 2016;29(4):306-8. [ Links ]
4. Germain MJ, Davison SN, Moss AH. When enough is enough: the nephrologist´s responsibility in ordering dialysis treatments. Am J Kindey Dis. 2011;58(1):135. [ Links ]
5. Davison SN. The ethics of end-of-life care for patients with ESRD. Clin J Am Soc Nephrol. 2012; 7:2049-57. [ Links ]
6. Brennan F, Stewart C, Burgess H, Davison SN, Moss AH, Murtagh F.E.M., et al. Time to improve informed consent for dialysis: an international perspective. Clin J Am Soc Nephrol. 2017; pii: CJN.09740916. [ Links ]
7. Jaufman SR, Shum JK, Russ AJ. Older age, life extension, and the character of medical choice. J Gerontol B Psychol Sci Soc. 2006; 61: S175-84. [ Links ]
8. Hines SC, Badzek L, Moss AH. Informed consent among chronically ill elderly: assessing its (in)adequacy and predictors. J Appl Commun Res. 1997; 25:151-69. [ Links ]
9. Davison SN: End-of-life preferences and needs: perceptions of patients with chronic kidney disease. Clin J Am Soc Nephrol 2010; 5:195-2014. [ Links ]
10. Kerr PG. Hemodialysis practices: new trends and associations with outcomes in the international Dialysis Outcomes and Practice Patterns Study. Presented at American Society of Nephrology Renal Week, November 19, 2010, Philadelphia PA. [ Links ]
11. Teruel JL, Vion VB, Couto AG, Gorrín MR, Fernandéz-Lucas M, Mendiola NR, et al. Choosingconservativetherapy in chronic Kidney disease. Nefrologia. 2015; 35(3):273-9. [ Links ]
12. Fung E, Slesnick N, Tamura MK, Schiller B. A survey of views and practice patterns of dialysis medical directors toward end-of-life decision making for patients with end-stage renal disease. Palliat Med. 2016; 30(7):653-60. [ Links ]
13. Biesen WV, Van de Luijtgaarden MWM, Brown E, Michel JP, Munster BC, Jager KJ, Van der Veer SN. Nephrologist´s perceptions regarding dialysis withdrawal and palliative care in Europe: lessons from a European Best Practice survey. Nephrol Dial Transplant. 2015;30:1951-58. [ Links ]
14. Murtagh FE, Sheerin N. Conservative management of end stage kidney disease. In: Chambers JE Brown EA, Germain MJ, eds. Supportive care for the renal patients. 2nd ed. Oxford, UK: Oxford University Press; 2010:53-268. [ Links ]
15. Quill TE, Holloway R. Time-limited trials near the end of life. JAMA 2011;306:1483-4. [ Links ]
16. Rinehart A. Beyond the Futility Argument: The Fair Process Approach and Time-Limited Trials for Managing Dialysis Conflic. Clin J Am Soc Nephrol. 2013;8:2000-6. [ Links ]
17. Wightman A. Management dilemmas in pediatric nephrology: time-limited trials of dialysis therapy. Pediatr Nephrol. 2017;32(4):615-20. [ Links ]
18. Rosansky SJ, Cancarini G, Clarck WF, Eggers P, Germain M, Glassock R, et al. Dialysis initiation: what´s the rush? Semin Dial. 2013;26(6):650-7. [ Links ]
19. O´Hare AM, Choi AI, Berthenthal D, et al. Age affects outcome in chronic kidney disease. J Am Soc Nephrol. 2007;18:2758-765. [ Links ]
20. Brown EA, Finkelstein FO, Iyasere O, Kliger AS. Peritoneal or hemodialysis for the frail elderly patient, the choice of two evils? Kidney Int. 2017;91(2):294-303. [ Links ]
21. Berger, JR, Jaikaransigh V, Hedayati SS. End-stage kidney disease in the elderly: approach to dialysis initiation, choosing modality and predicting outcomes. Adv Chronic Kidney Dis. 2016;23(1):36-43. [ Links ]
22. Harris SAC, Lamping DL, Brown EA Constantinovici N, for the NTDS group. Dialysis modality and elderly people. Effect on clinical outcomes and quality of life. Perit Dial Int. 2002;22:463-70. [ Links ]
23. Brown EA, Johansson L, Farrington K, et al. Broadening options for long-term dialysis for the elderly (BOLDE): differences in quality of life on peritoneal dialysis compared to hemodialysis for older patients. Nephrol Dial Transplant. 2010;25:3755-63. [ Links ]
24. National Kidney Foundation. KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis. 2015;66:884-930. [ Links ]
25. Hall YN, Larive B, Painter P, Kaysen GA, Lindsay RM, Nissenson AR, et al. Effects of frequent hemodialysis on physical performance, health and functioning: frequent hemodialysis network trials. Clin J Am Soc Nephrol. 2012;7:782-94. [ Links ]
26. Grubbs V, Moss AH, Cohen LM, Fisher MJ, Germain MJ, Jassal SV, et al. A palliative approach to dialysis care: a patient-centered transition to the end of life. Clin J Am Soc Nephrol. 2014 Dec 5;9(12):2203-9 [ Links ]
27. Davison SN, Jassal SV. Supportive care: integration of patient-centered kidney care to manage symptoms and geriatric syndromes. Clin J Am Soc Nephrol. 2016;11(10):1882-91. [ Links ]
28. Douglas CA. Palliative care for patients with advanced chronic kidney disease. JR Coll Physicians Edinb. 2014;44:224-31. [ Links ]
29. Farinha A. Symptom control in End Stage Renal Disease. Port J Nephrol Hypert. 2017;31(3):192-9. [ Links ]
30. Gong S, Sheng P, Jin H, Qi E, Chen W, Dong Y, et al. Effect of methylphenidate in patients with cancer-related fatigue: a systematic review and meta-analysis. PLoS One. 2014;9(1):e84391. [ Links ]
31. O´Connor NR, Corcoran AM. End-stage renal disease: symptom management and advance care planning. Am Fam Physician. 2012;85(7):705-10. [ Links ]
32. The 2009 NHS Kidney Care and the National. End of Life in Advanced Kidney Disease – A Framework for Implementation. [ Links ]
Carolina Lã Belino, MD
Department of Nephrology, CHVNG/E, Conceiçã o Fernandes Street
4434-502, Vila Nova de Gaia, Portugal.
E-mail: carolinabelino@hotmail.com
Acknowledgments
We like to thank to Prof. Manuel Pestana for all the knowledge, support and availability provided.
Support: No financial or non-financial support of any kind was provided.
Disclosure of potential conflicts of interest: The authors disclose no conflicts of interest
Received for publication: Nov 11, 2017
Accepted in revised form: May 30, 2018














