Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.32 no.1 Lisboa mar. 2018
CASE REPORT
Prostate involvement in granulomatosis with polyangiitis: response to rituximab treatment
David Navarro1, Charles Pusey2
1 Nephrology Department, Centro Hospitalar de Lisboa Central E.P.E., Hospital Curry Cabral, Lisbon, Portugal.
2 Renal and Vascular Inflammation Section, Department of Medicine, Imperial College London, London, UK.
ABSTRACT
Granulomatosis with polyangiitis is an autoimmune vasculitic condition strongly associated with anti-neutrophil cytoplasm antibodies. It classically affects the respiratory tract and the kidney, but it can manifest in a multitude of other organs. Urological involvement is uncommon and can be difficult to diagnose. We present two cases of GPA with prostatic involvement, with distinct clinical manifestations. Both cases responded well to treatment with rituximab.
Keywords: ANCA; Crescentic glomerulonephritis; prostate; Rituximab; Vasculitis.
INTRODUCTION
Granulomatosis with polyangiitis (GPA, previously Wegeners granulomatosis) is an autoimmune vasculitic condition that classically involves the upper and lower respiratory tract and the kidney. A multisystem disease, it can manifest in other sites, causing gastrointestinal, cardiac, musculoskeletal, ocular or neurological disease1.
GPA is strongly associated with anti-neutrophil cytoplasm antibodies (ANCA), and forms part of the spectrum of ANCA-associated vasculitis (AAV).
The prostate presentations of GPA are diverse, and can include prostatitis, chronic obstructive urinary symptoms, acute urinary retention, macroscopic haematuria and recurrent urinary tract infections, or can be asymptomatic2-4. Urogenital involvement is considered uncommon with an incidence of 7.4% in an early autopsy study5. Urogenital manifestations diagnosed in life are rare, as reflected by the 0.7% incidence in a recent series by the French Vasculitis Study Group6. However, this contrasts with the 10% incidence of urogenital involvement reported by Huong7. This discrepancy might be partially explained by lack of symptom recognition, which is of importance since urogenital complaints may be the first manifestation of GPA6. There are no clear diagnostic criteria for prostate involvement and a high level of clinical suspicion is necessary.
Although standard treatment for GPA has been prednisolone and cyclophosphamide, rituximab has been shown to be an alternative approach8-10, in particular in cases where there is frequent recurrence or fear of cyclophosphamide toxicity. We present two cases of GPA with prostatic involvement, with distinct clinical manifestations. Both cases responded well to treatment with rituximab.
CASE REPORT
Case 1
A 63-year old Caucasian man presented in 2012 with acute kidney injury (serum creatinine 2.83 mg/dL), haematuria and lung nodules detected on CT-scan. The kidney biopsy showed crescentic glomerulonephritis and ANCA was positive with anti-PR3 antibodies of 125 U/ml (N<25). He was started on steroids, cyclophosphamide and rituximab, with good clinical response, and two months later his serum creatinine was 1.76 mg/dL and ANCA became negative. Although he was kept on maintenance therapy with azathioprine and prednisolone, he had two relapses with eye involvement, both treated successfully with rituximab.
In 2015, due to recurrent haematuria, frequency, nocturia and poor urinary flow, there were concerns about urological malignancy given his previous cyclophosphamide exposure, and he was referred to Urology.
PSA level was normal at 1μg/L (N <4) and anti-PR3 antibodies had risen to 75 IU (NR <3). Urine culture was negative, and urinary dipstick was negative for nitrites, despite containing some leukocytes. Ultrasound scan showed a prominent prostate, although there was no significant post-micturition volume. Subsequent flexible cystoscopy showed normal urethra and an occlusive prostate, but no suspicious lesions. A transurethral resection of the prostate (TURP) was performed, and histological analysis showed features of GPA (Figure 1).
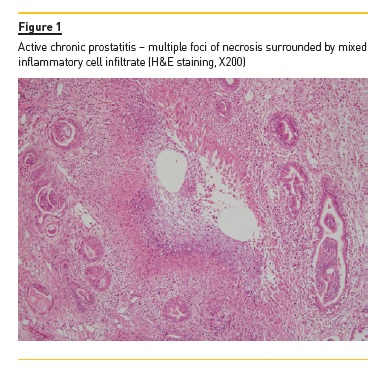
Since there was only moderate improvement after TURP, the patient was treated with an increase in prednisolone dose and with rituximab, to avoid further cyclophosphamide exposure. The patients symptoms rapidly improved, with only occasional haematuria and resolution of lower urinary tract symptoms (LUTS). The patient continues to be followed the vasculitis clinic – he is under rituximab maintenance therapy and there has been no signs of disease relapse.
Case 2
A 30-year-old Indian man with history of epilepsy and a non-functioning pituitary microadenoma, was diagnosed in 2007 with GPA on the basis of ocular disease – scleritis and lachrymal gland vasculitis – and PR3-ANCA positivity of 420 U/ml (N <25). He had no evidence of renal involvement, and was treated initially with mycophenolate mofetil (MMF) and prednisolone.
Due to persistent symptoms and raised intra-ocular pressure (felt to be due to steroid therapy), rituximab was added, with excellent clinical response. Maintenance immunosuppression consisted of MMF and prednisolone, and sustained remission allowed for progressive weaning of immunosuppressive treatment, which was stopped in 2009.
In 2014, the patient started complaining of LUTS, haematuria and hematospermia, and was referred to Urology. Magnetic resonance imaging of the prostate was reported to show a prostatic abscess, and the computed tomography urogram showed an enlarged prostate without signs suspicious of tumor. Meanwhile, he remained otherwise well, with no evidence of recurrent eye disease. PSA was normal at 2μg/L (N <4) and ANCA became positive with anti-PR3 antibodies of 11 IU (NR < 3). Despite several courses of antibiotics, the patients symptoms persisted. A trans-rectal guided biopsy of the prostate showed signs of active chronic prostatitis with abundant necrosis – albeit without granulomas – consistent with GPA. Since he started to feel generally unwell, a trial of prednisolone was attempted. The patients symptoms improved rapidly, and rituximab was administered, given his historic favorable response to this agent. Maintenance therapy consisted of prednisolone, which was progressively weaned and stopped in mid-2015. The patient has since been asymptomatic, and remains under follow-up in the vasculitis clinic.
DISCUSSION
We describe two patients with known GPA who developed clinically significant prostatic disease. The first case developed predominant symptoms of bladder outflow obstruction accompanied by haematuria. In view of previous cyclophosphamide exposure, it was suspected he might have developed a bladder or prostate malignancy. It was only when he underwent prostatic biopsy at the time of TURP that the diagnosis of GPA of the prostate became clear. The second case developed symptoms similar to those of prostatitis, with pain, haematuria and haematospermia. Although imaging suggested a prostatic abscess, biopsy of the prostate confirmed the diagnosis of GPA.
The difficulty in diagnosing prostatic involvement in GPA is illustrated by these two cases, in both of which the diagnosis took many months to establish. In both cases, prostatic disease was the only new organ involvement, although the second patient also felt generally unwell. Clinicians need to be aware of the possibility of prostate involvement in patients with GPA in order to avoid diagnostic delay. Diagnosis is even more difficult in those rare cases of GPA where prostate involvement is the first disease manifestation. Of note, despite significant prostatic symptoms attributed to GPA, ANCA can be negative; in a previous review, 19% of GPA patients with urogenital involvement were ANCA negative6.
Both patients were treated successfully with rituximab together with corticosteroids, as both had responded well to rituximab in the past. Furthermore, rituximab was used in the first patient to avoid further cyclophosphamide, and in the second case due to patient preference. Although conventional treatment for induction of remission in GPA includes prednisolone and cyclophosphamide, two recent randomized controlled trials demonstrated that rituximab was noninferior to cyclophosphamide8,9.
Rituximab is selected in frequently relapsing disease, and also in cases where cyclophosphamide is relatively contraindicated, or where the patient prefers to avoid it. Surgical procedures, such as TURP, might be necessary in patients with persistent symptoms after receiving immunosuppressants; this was the case in 55% of patients in a recent series6. In conclusion, although rare, prostate involvement in GPA is probably underestimated because of its wide spectrum of manifestations.
Urogenital involvement should therefore be considered in all male GPA patients presenting with LUTS.
Rituximab seems to be a safe and effective therapeutic option in these patients, requiring confirmation in further studies.
References
1. Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1-11. [ Links ]
2. Middleton G, Krap D, Lee E, et al. Wegeners granulomatosis presenting as a lower back pain with prostatitis and urethral obstruction. J Rheumatol. 1994;21(3):566-9. [ Links ]
3. Davenport A, Downey SE, Goel S, Maciver AG. Wegeners granulomatosis involving the urogenital tract. Br J Urol. 1996;78(3):354-7. [ Links ]
4. Khattak AQ, Nair M, Haqqani MT, Williamson EPM. Wegeners granulomatosis: prostatic involvement andrecurrent urinary tract infections. BJU Int. 1999;84(4):531-2. [ Links ]
5. Walton EW. Giant-cell granuloma of the respiratory tract (Wegeners granulomatosis). Br Med J. 1958;2(5091):265-70. [ Links ]
6. Dufour JF, Le Gallou T, Cordier JF et al. Urogenital manifestations in Wegener granulomatosis a study of 11 cases and review of the literature. Medicine. 2012;91(2):67-74. [ Links ]
7. Huong DL, Papo T, Piette JC et al. Urogenital manifestations of Wegener granulomatosis. Medicine. 1995;74(3):152-61. [ Links ]
8. Jones RB, Tervaert JW, Hauser T et al. Rituximab versus cyclophosphamide in ANCAassociated renal vasculitis. N Engl J Med. 2010;363(3):211-20. [ Links ]
9. Stone JH, Merkel PA, Spiera R et al. Rituximab versus cyclophosphamide for ANCAassociated vasculitis. N Engl J Med. 2010;363(3):221-32. [ Links ]
10. Yates M, Watts RA, Bajema IM et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis. 2016;75(9):1583-94. [ Links ]
David Navarro, MD
Nephrology Department
Centro Hospitalar Lisboa Central E.P.E., Hospital Curry Cabral
Rua da Beneficência 8, 1069-166 Lisboa, Portugal
E‑mail: davidbnavarro@gmail.com
Acknowledgements
We acknowledge support from the NIHR Imperial Biomedical Research Centre. We thank Prof. Terence Cook for providing histology images for case 1.
Disclosure of potential conflicts of interest: none declared
Received for publication: Jan 14, 2018
Accepted in revised form: Jan 30, 2018














