Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Portuguese Journal of Nephrology & Hypertension
Print version ISSN 0872-0169
Port J Nephrol Hypert vol.31 no.4 Lisboa Dec. 2017
ORIGINAL ARTICLE
Predicting early mortality in incident hemodialysis patients: strengthening a shared decision-making process
Carolina L. Belino1,2,3, Augusto M. Coelho2,3, Susana J. Pereira1,2,3, Daniela M. Lopes1,2,3, C. Silva4, Ana M. Gomes1,2,3, Ana M. Ventura1,2,3
1 Department of Nephrology, Centro Hospitalar Vila Nova de Gaia e Espinho, Vila Nova de Gaia, Portugal
2 Hemodialysis Center Hemoatlântico Gaia, Vila nova de Gaia, Portugal
3 Diaverum, Vila nova de Gaia, Portugal
4 Statr.analysis Company, Statistics, Vila Nova de Gaia, Portugal
ABSTRACT
Introduction: The benefits of dialysis in the elderly are dubious. A shared decision‑making process, helped by adequate prognostic tools, is essential to determine which patients are better candidates for conservative care.
Based on a recent USRDS validated score, this study aimed to identify risk factors associated with early mortality (first 90 days) in a Portuguese cohort of patients.
Methods: A total of 197 patients who initiated hemodialysis treatments in a Portuguese facility between 2005 and 2015 were included. Clinical and laboratory data were collected at time of admission to center. Multiple regression models were performed and fitted to identify potential predictors of early mortality.
Findings: Total of 93 (47.2%) deaths with 23 (11.7%) deaths occurring in first year. In the first three months, there were 15 (7.6%) deaths. Of those who died in first three months, most were men (n=10; 5.1%), mean age 73.5 ± 6.82 years. Almost half (n=7; 3.6%) were dependent and the majority (n=12; 6.1%) had history of hospitalizations in previous year before admission. They had a higher prevalence of hypoalbuminemia and cardiovascular risk factors. Mortality associated factors were albumin level low (<3.5 g/dL) or unknown (OR 5.73; p<0.05), ischemic cardiomyopathy (OR 4; p<0.05) and history of hospitalizations in previous year before admission (OR 4.3; p<0.05). Absence of history hypertension was associated with a reduction of risk (OR 0, 18, p<0.05).
Discussion: Some elements of USRDS score were associated with greater risk for early mortality in this Portuguese cohort of patients. Further investigations are needed in order to validate a specific prognostic tool in Europeans.
Keywords: elderly, conservative care, mortality risk factors, prognostic tools
INTRODUCTION
Chronic kidney disease already affects 10% of the population worldwide. Prevalence can vary from 11.8% in United States to 17.3% and 18.3% in Europe and China, respectively1.
Numbers are expected to increase disproportionally in countries where the percentage of elderly people is growing1‑4.
Older patients (≥ 65 years) are the fastest‑growing segment of the incident end‑stage renal disease (ESRD) population and have nearly doubled since 19973. A 57% age‑adjusted increase in dialysis for octogenarians and nonagenarians was documented in the United States between 1996 and 20035,6. Currently, more than half of patients initiating dialysis are > 60 years of age6. However, evidence from the last decade has revealed that overall benefit of dialysis in these age segments is dubious and may be detrimental to survival and quality of life6,7.
Nephrologists around the world face the challenge of determining which patients are more appropriate for conservative management as an alternative to dialysis, raising multiple moral and ethical questions.
The Renal Physician Association guideline on appropriate initiation and withdrawal of dialysis recommend an estimation of survival chances for all patients requiring dialysis. Numerous scores for mortality prediction have been developed in last decade but only a few of them have focused on short‑term survival (≤ 6 months). The French REIN registry8 and the New England HD clinics score9 are robust and proper validated tools for 6‑month survival estimates10.
Recently, Thamer and his colleagues validated a simple 3‑month survival score after dialysis start using the US Renal Data System, which considered data from 69,441 patients with age ≥ 67 years11. It uses routinely and readily available information that can be used by patients, families and their nephrologists to estimate the risk of early mortality after starting dialysis. In comparative analysis to UK and French models, it has been shown to have the best model fit and discrimination capacity11.
Considering this study, we aimed to investigate early mortality risk factors in a Portuguese cohort of hemodialysis patients.
MATERIALS AND METHODS
Study population, Setting, and Design
Data from all patients who initiated hemodialysis (HD) treatments in a Portuguese facility between January 2005 and December 2015 were collected using the facility system. Sociodemographic data, comorbid information and medical history, health resource use, institutionalization, dialysis‑related information and laboratory data were retrieved for each patient at time of center admission.
Specifically, 273 patients initiated hemodialysis treatments in this center. Of these, 76 patients were excluded because of missing information. A total of 197 patients were included. The possible dialysis modalities were high flux hemodialysis, hemodiafiltration and nocturnal hemodialysis.
End‑bpoints and Risk‑Factor Predictors
Primary goal was to analyze all‑cause mortality within the first 3 months, since there is a known mortality peak in this period, especially in elderly and severe ill patients5‑9.
Mortality within first 6 months was not selected as end‑point since there were only four deaths in this period.
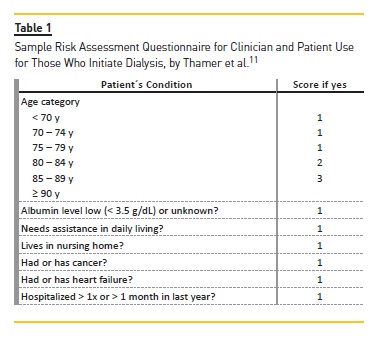
Variables chosen as potential predictors of early mortality were based on the Thamer study (Table I) and were age, gender, history of hypertension, cerebrovascular disease, ischemic cardiopathy, heart failure, diabetes mellitus, cancer, albumin level low (<3.5 g/dL) or unknown, assistance in daily living activities, residence in nursing home and history of hospitalization more than one time or more than a month in the previous year before center admission.
Statistical Analysis
Baseline characteristics were analyzed by standard descriptive statistics. The Kolmogorov‑Smirnov test was used to test normality distribution of variables. Patients were divided into two cohorts: those who died within 3 months after starting dialysis and those who didn´t.
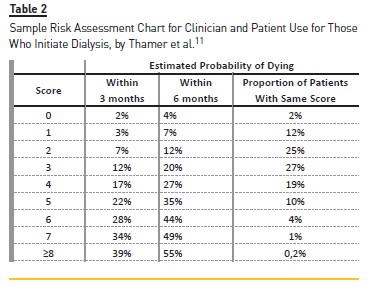
A simple comparison was made with respective US Renal Data System cohorts. Posteriorly, a univariate analysis was made. The variables considered were included as possible 3‑month mortality predictors in multiple logistic regression with backward elimination method used to define the final risk predictors. We also applied the Sample Risk Assessment Chart used by Thamer et al.11 to examine the proportions of patients and deaths by score categories (Table 2).
RESULTS
Median age was 71 ± 15.5 years. Patient characteristics are summarized in Table 3. In total, there were 93 (47.2%) deaths. Only 23 (11.7%) of them occurred in the first year. The first three months was the period with a higher density of deaths (n=15; 7.6%).
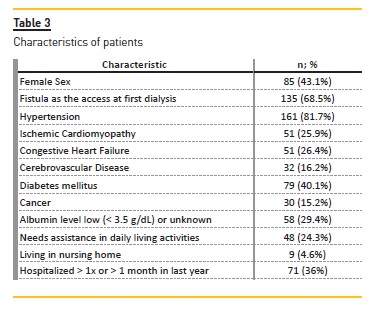
A comparison with USRDS cohort is made in table 4. Between two groups, major differences were in the higher prevalence of central venous catheter as first vascular access, diabetes mellitus, ischemic cardiomyopathy, hospitalizations and institutionalization in those who died within 3 months.
In US Renal Data System groups, patient characteristics were similar, except in institutionalization rates and first vascular access, with catheter also being more frequent in those who died within 3 months.
After applying the Sample Risk Assessment Chart (Table 5), a great majority of patients were categorized between categories 0 and 4, corresponding to an estimated probability of dying at 3 months of 2% and 7%, respectively.
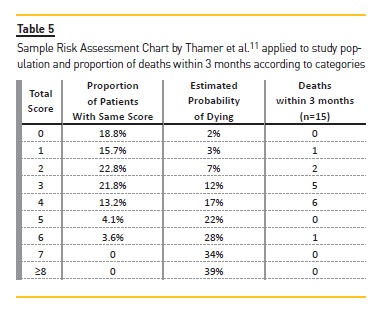
The predominance was for categories 2 (22. 8%) and 3 (21. 8%), corresponding to a low/intermediate risk of dying. However, most of deaths in the first 3 months (n=13; 6.6%) occurred in patients categorized as 3 and/or 4.
Risk factors for mortality
Table 6 shows the multiple regression analysis. All variables of the simple model had p< 0.05. The absence of hypertension was associated with a reduction of risk (OR 0.18, p<0. 05).
The variables associated with greater risk of global mortality were albumin level low (<3.5 g/dL) or unknown (OR 5.73; p<0.05), ischemic cardiomyopathy (OR 4; p<0.05) and history of hospitalizations in previous year before admission (OR 4.3; p<0.05). Absence of history of hypertension was associated with a reduction of risk (OR 0, 18, p<0.05).
DISCUSSION
International data shows high mortality in elderly patients starting dialysis, with highest rates in the United States and Australia/New Zealand13. Causes are multifactorial and related to policies accepting polymorbid and/or advanced age and dependent patients. Some of these patients had experienced renal failure secondary to a systemic illness were already in an active dying process in which dialysis may not alter the course12,13.
Further to this, there are more debilitating symptoms and geriatric syndromes such as cognitive dysfunction or sensory impairments as well as functional and psychological dependence. Dialysis can impose additional burdens, including invasive procedures. Rehabilitation does not lead to great results and prognosis is generally poor9‑13.
In one study, 63% of patients who decided to initiate dialysis therapy regretted this choice; 52% indicated they initiated therapy by physician´s recommendation12. Only 39% of 3702 nursing home ESRD patients with functional impairment maintained baseline function at 3 months after dialysis initiation, decreasing to 13% at 3 months14.
Many older patients on maintenance dialysis in the United States continue to receive intensive care focused on life prolongation instead of decreasing pain and suffering.
About 45% die in hospital setting compared with 35% of patients with other chronic diseases, such as congestive heart failure, dementia or advanced liver disease.
During the final month, rates of hospitalization (76%) and intensive care unit admission (49%) are higher than reported for cancer patients15,16. However, services of palliative care are extremely low, about 20%, compared with terminal cancer (55%) and heart failure (39%) patients16‑18.
Only 18% expressed preference to live as long as possible, independent of suffering19. Similarly, in Canada, only 18% favored dialysis to prolong their lives and there were more patients wishing to die at home (36%) or in an inpatient hospice (29%) than in a hospital (27%)20. Also, dialysis represents one of the most expensive public‑financed treatments, costing billions per year. Appropriate resource allocation is fundamental21.
Taking all this into account, its fundamental to identify who benefits from dialysis, when to start dialysis and what the ideal modality is. This is now instituted as a priority in developed countries22‑25.
Before the release of the IDEAL trial23, elderly were starting dialysis at higher GFRs. It has been suggested that the severity of renal disease may be overdiagnosed based on method inaccuracies and the elderly probably have slower progression to ESRD, being most likely to die than progress to ESRD. Conservative (nondialytic) care is widely provided but, until recently, it has not been clearly defined and multiple alternative terms have been applied, preventing adequate studies in this field23. The recent KDIGO conference on supportive care in chronic kidney disease24 proposed the term comprehensive conservative care, a holistic approach patient‑centered, focused on patient goals, relief of suffering, preservation of functional status and quality of life12,22. The shared‑decision making process assumes a central role, in which clinicians, patients and families join together to consider the best medical evidence in light of patients characteristics and values when choosing health care26.
Evidence on conservative care is limited but its reasonably clear that dialysis is associated with a significant survival advantage but its markedly reduced for older (> 75 years) and polymorbid patients (especially ischemic cardiomyopathy) or with poor functional status. Quality of life, symptoms and hospital‑free survival must be considered22.
Information about patients on peritoneal dialysis (PD) is scarce. PD patients are younger, fitter and more independent so comparisons are difficult. Also, the elderly that are more severe ill and dependent are more likely to receive HD as a most suitable modality27. Further studies are needed to clarify the contribution of each dialysis modality into prognosis.
The patient‑specific estimate of prognosis is recommended to facilitate informant consent and to support the discussion of care goals23,24. Numerous scores of mortality on dialysis have been developed over the course of the last decade. Scores are integrated prognostic scoring systems that include clinical, biological and other characteristics which when applied to risk estimation are designated as prognostic tools24.
Although they are not sensitive or specific to predict with certainty how well a patient will do or how long he/she will live, they help to identify high‑risk patients who can be targeted for specific interventions or alternative care pathways.
However, the process of score creation is hard and complex, due to the multiple comorbidities and overlap diagnoses. A recent national study in Canada26 revealed that more than 80% of nephrologists were not satisfied with their capacity to predict clinical trajectories.
Current tools validated to predict short‑term mortality are the US Renal Data System score, the French REIN registry score, the Catalan renal registry score and the New England HD clinic score22.
The US Renal Data System score, by Thamer et al.11, is the largest study that included more variables on functional status and dependence. It is addressed to patients, clinicians and society and has a good calibration and discrimination parameters. As far as we know, this is the first non‑American study seeking to reproduce similar results in order to establish foundations for posterior investigation and validation in Europe.
Our study was limited by the small sample size and by having few mortality events in the considered time.
This is the probable reason why further risk associations with the other elements of USRDS score were not found.
The retrospective design limits the accuracy in data collection and correction for residual confounders. Also, there were no specific inclusion criteria other than the beginning of hemodialysis treatments in this facility.
We didn´t take into consideration the effect of different dialysis modalities in survival rates. Nocturnal dialysis was a recent option and hemodia filtration was not always available.
However, we were able to confirm the importance of functional status and recent hospitalizations, as a marker of frailty, in mortality and prognosis. We identified a new element, no history of hypertension, as a positive prognostic marker. Hypertension is intimately linked to cardiovascular disease and cardiomyopathy and cardiovascular diseases are the main cause of death in these patients, so no past history of hypertension may be an indicator of significantly less severe disease and as a consequence of that, better outcome.
CONCLUSIONS
The elderly is the fastest growing segment of incident and prevalent patients on dialysis. They have more disability, symptom burden and comorbidities and their quality of life and prognosis are poorer. Conservative care programs have shown benefits and have to be adequately explored in the right patients, through a shared decision‑making process, for which prognostic tools are fundamental. There are several validated scores and the US Renal Data System score is a good candidate with focus on disability and functional status.
It seems that some of the elements of this score are suitable for European patients, but further investigations are needed in order to design and validate a specific prognostic tool.
References
1. Brück K, Stel VS, Gambaro G, Hallan G, Völzke H, Ärnlöv J, et al. CKD prevalence varies across the european general population. J Am Soc Nephrol 2016 Jul;27(7):2135–2147. [ Links ]
2. National Kidney Foundation: Global facts about kidney disease [internet]. [Accessed August 19, 2016]. Available from: https://www.kidney.org/kidneydisease/global‑facts‑about‑kidney‑disease. [ Links ]
3. Jha V, Garcia‑Garcia G, Iseki K, Li Z, Naicker S, Plattner B,et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013 Jul;382(9888):260–272. [ Links ]
4. Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011 Dec;80(12):1258–1270. [ Links ]
5. Thorsteinsdottir B, Swetz KM, Albright RC. The ethics of chronic dialysis for the older patient: time to reevaluate the norms. Clin J Am Soc Nephrol 2015 Nov;10(11):2094–2099. [ Links ]
6. Davison S. The ethics of end‑of‑life care for patients with ESRD. Clin J Am Soc Nephrol 2012 Dec;7(12):2049–2057. [ Links ]
7. Grubbs V, Moss A, Cohen L, Fisher MJ, Germain MJ, Jassal SV, et al. A palliative approach to dialysis care: a patient centered transition to the end of life care. Clin J Am Soc Nephrol 2014 Dec;9(12):2203–2209. [ Links ]
8. Couchoud C, Labeeuw M, Moranne O, Allot V, Esnault V, Frimat L, et al. A clinical score to predict 6‑month prognosis in elderly patients starting dialysis for end‑stage Renal disease. Nephrol Dial Transplant 2009 May;24(5):1553–1561. [ Links ]
9. Cohen LM, Ruthazer R, Moss AH, Germain MJ. Predicting six‑month mortality for patients who are on maintenance hemodialysis. Clin J Am SocNephrol. 2010 Jan; 5(1):72–79. [ Links ]
10. Couchoud C, Hemmelgarn B, Kotanko P, Germain MJ, Moranne O, Davidson S. Supportive care: time to change our prognostic tools and their use in CKD. Clin J Am Soc Nephrol 2016 Oct 7;11(10):1892–1901. [ Links ]
11. Thamer M, Kaufman JS, Zhang Y, Zhang Q, Cotter DJ, Bang H. Predicting early death among elderly dialysis patients: development and validation of a risk score to assist shared decision making for dialysis initiation. Am J Kidney Dis. 2015 Dec;66(6):1024–1032. [ Links ]
12. Germain M, Davison S, Moss A. When enough is enough: the nephrologist´s responsibility in ordering dialysis treatments. Am J Kidney Dis. 2011 Jul;58(1):135–143. [ Links ]
13. Iyasere O, Brown E, Johansson L, Huson L, Smee J, Maxwell AP, et al. Quality of life and physical function in older patients on dialysis: a comparison of assisted peritoneal dialysis with hemodialysis. Clin J Am Soc Nephrol 2016 Mar;11:423–430. [ Links ]
14. Kurella TM, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med 2009 Oct;361(16):1539–1547. [ Links ]
15. Grubbs V, Moss AH, Cohen LM, Fisher MJ, Germain MJ, Jassal SV. A palliative approach of dialysis care: a patient‑centered transition to the end of live. Clin J Am Soc Nephrol 2014 Dec;9(12):2203–2209. [ Links ]
16. Wong SP, Kreuter W, O´Hare AM. Treatment intensity at the end of life in older adults receiving long‑term dialysis. Arch Intern Med 2012 Apr;172(8):661–664. [ Links ]
17. Murray AM, Arko C, Chen SC, Gilbertson DT, Moss AH. Use of hospice in the united states dialysis population. Clin J Am Soc Nephrol 2006 Nov;1(6):1248–1255. [ Links ]
18. O´Connor NR, Dougherty M, Harris PS, Casarett DJ. Survival after dialysis discontinuation and hospice enrollment for ESRD. Clin J Am Soc Nephrol 2013 Dec;8(12):2117–2122. [ Links ]
19. Hines SC, Glover JJ, Babrow AS, Holley JL, Badzek LA, Moss AH. Improving advanced care planning by accommodating family preferences. J Palliat Med. 2001 Winter;4(4):481–489. [ Links ]
20. Davidson SN. End of life preferences and needs: perceptions of patients with chronic kidney disease. Clin J Am Soc Nephrol 2010 Feb;5(2):195–204. [ Links ]
21. Morton R, Tamura MK, Coast J, Davison SN. Supportive care: economic considerations in advanced kidney disease. Clin J Am Soc Nephrol 2016 Oct;11(10):1915–1920. [ Links ]
22. Murtagh FE, Burns A, Moranne, Morton RL, Naicker S. Comprehensive conservative care in end‑stage kidney disease. Clin J Am Soc Nephrol 2016 Oct;11(10):1909‑1914.
23. Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Fraenkel MB, et al. A randomized controlled trial of early versus late initiation of dialysis. N Engl J Med 2010 Aug 12;363(7):609–619. [ Links ]
24. Davison SN, Levin A, Moss AH, Jha V, Brown EA, Brennan F, et al. Kidney Disease: Improving Global Outcomes: executive summary of the KDIGO controversies conference on supportive care in chronic kidney disease: developing a roadmap to improving quality care. Kidney Int 2015;88:447–459. [ Links ]
25. Moss AH. Shared decision –making in dialysis: the new PRA/ASN guideline on appropriate initiation and withdrawal of treatment. Am J Kidney Dis 2001 May;37(5):1081–1091. [ Links ]
26. Chiu HH, Tangri N, Djurdjev O, Barrett BJ, Hemmelgarn BR, Madore F, et al. Perceptions of prognostic risks in chronic kidney disease: a national survey. Can J Kidney Health Dis 2015 Dec;2:53. [ Links ]
27. Da Silva‑Gane M, Wellsted D, Greenshields H, Norton S, Chandna SM, Farrington K. Quality of life and survival with advanced kidney failure managed conservatively or by dialysis. Clin J Am Soc Nephrol 2012 Dec;7(12):2002–2009. [ Links ]
Carolina Lã Belino, MD
Department of Nephrology
Centro Hospitalar Vila Nova de Gaia e Espinho
Rua Conceição Fernandes 4434‑502,
Vila Nova de Gaia, Portugal
E‑mail: carolinabelino@hotmail.com
Disclosure of potential conflicts of interest: none declared
ACKNOWLEDGMENTS
We thank Thamer and colleagues for permission to use material from their work in our study. All authors played a part in the drafting of the manuscript, search strategy, data extraction and results interpretation and discussion. All contributed with medical expertise on hemodialysis and on methodology. CB performed the bibliographic research and wrote the manuscript. CS performed the statistical analysis.
Received for publication: Oct 2, 2017
Accepted in revised form: Nov 15, 2017














