Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.31 no.3 Lisboa set. 2017
REVIEW ARTICLE
Symptom control in End Stage Renal Disease
Ana Farinha
Nephrology Department, Centro Hospitalar de Setúbal, Portugal
ABSTRACT
Chronic kidney disease (CKD) patients experience a high burden of symptoms that are not always recognized or relieved by standard measures. Principles of palliative care may be helpful both in dialysis or conservative treatment. This article intends to review some of the most common and distressing symptoms affecting ESRD patients in daily life, focusing on pathogenesis, pharmacological and non-pharmacological management. It will not include end of life care.
INTRODUCTION
Until the twentieth century, medical care was based on relief of symptoms. Diseases followed their natural courses: etiologies and physiopathology were not known, so cure could not be expected. Over the last century this paradigm changed and medicine has focused on disease treatment, relegating symptom control to second place.Chronic kidney disease (CKD) is a chronic disease with a higher burden of symptoms, particularly in end stage renal disease (ESRD)1. Dialysis may provide a major benefit but it will not ameliorate or abolish all symptoms and may even contribute to some of them.
SYMPTOM ASSESSMENT
Doctors cannot treat what they cannot recognize.
Most patients do not discuss symptoms with their doctors and most doctors do not ask their patients. The routine use of instruments to check symptoms, on a regular basis, is important to recognize symptoms that otherwise may be underestimated or forgotten. The renal version of the Patient Outcome Scale (POSs renal)2 or Modified Edmonton Symptom Assessment Scale4 (ESAS)3 are two examples of scales adjusted for CKD patients that assess most common symptoms in this context. These tools also allow evaluation of the severity of symptoms, how they impact patients lives and how measures taken improve them.
After recognition, it is important to characterize the symptom. It is crucial to make a detailed clinical history (determinate duration, worsening or improving factors), an objective examination and, if needed, complementary exams to find the cause. When treatment of the underlying cause has been exhausted, principles of palliative care should be applied: both pharmacological and non-pharmacological measures should be taken.
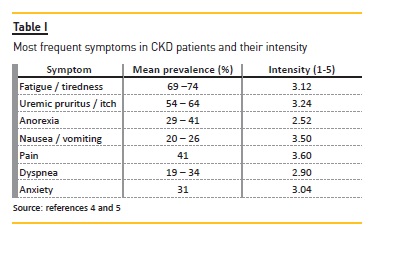
Most of these measures may not solve all the problems but they should improve quality of life. It is important to adjust expectations to a realistic level in order to avoid frustration that affects doctor-patient relationship and quality of life. The suggestions described below can be used by any nephrologist to any CKD patient, in conservative or dialysis management. In this specific population, the effect of dialysis must be considered because some drugs may be removed by dialysis. Some of these drugs are used in palliative care as off-label prescription. Most of these measures are not evidence based in CKD patients but have been extrapolated from other populations because studies in symptom control in CKD patients have not been addressed. The most common symptoms and their prevalence are summarized in Table I4–5. The most common drugs used in supportive management in ESRD patients are summarized in Table II.
FATIGUE/TIREDNESS
Fatigue is the most common symptom in ESRD in many studies4-9 with an estimated prevalence of about 70%. It is defined as lack of physical or mental energy, disproportionate to the activity level10. Etiology is often multifactorial but most theories are based on a change in cytokine profile. When assessing fatigue some common contributors must be considered since they are frequently involved:
– Anemia
– Sleep disorders
– Psychological or psychiatric disorders (e.g. anxiety, depression)
– Physical or psychological distress
– Uncontrolled symptoms (e.g. pain)
– Endocrine disorders (e.g. thyroid disorders, hypogonadism)
– Physical deconditioning related to prolonged immobilization
– Iatrogenic effects, usually in central nervous system
– Dehydration
– Infection
– Malnutrition with weight loss
– Concurrent illnesses (e.g. cardiac or pulmonary disease)
– Existential suffering (e.g. why me?)
In this context it is easy to understand that fatigue is one of the most challenging symptoms to manage. Both non-pharmacological and pharmacological measures should be used for better results. One of the most studied non-pharmacological measures is aerobic physical exercise adapted to functional capacity10. Physical deconditioning due to prolonged disease is given as an important cause of fatigue in a vicious circle where physiotherapy and/or occupational therapy should be promoted to break it. When fatigue persists despite this method, patients may also benefit from help with their daily activities, sparing energy for tasks that bring them pleasure or where they feel that they are really needed. Patients should review their schedule to perform activities during the moment of the day where they feel less tired.
Medications are of small benefit in fatigue but some have been studied. Steroids have been tried without a known mechanism of action. Dexamethasone in a dose of 6-8mg/day has been described to have a rapid onset (3 days) but it only may be used for a short period (2 weeks maximum). Side effects like edema, steroids myopathy or insulin resistance should be considered11.
Methylphenidate is one of the most promising drugs because of its effect as a stimulator of central nervous system. It has a rapid onset but it cannot be used for prolonged periods. It should not be used for longer than 8 weeks. It should be started in doses of 5-10mg up to 40-60mg, divided into two doses (breakfast and lunch).
Common collateral effects are activation of the Sympathetic Nervous System (SNS) presenting as agitation, tremors or tachycardia12. It should not be used in patients with ischemic myocardiopathy, arrhythmia, severe hypertension, hyperthyroidism or severe depression.
Modafinil is a drug that has been approved to treat narcolepsy. Although its mechanism of action remains elusive, it has been used to treat fatigue in terminal illnesses in doses from 100 to 400mg. The most problematic collateral effects are related to activation of the SNS12.
It is essential to treat the secondary causes of fatigue. Anemia may be the first cause that should excluded.
Nephrologists are aware of its impact. Erythropoiesis stimulators may be used to control anemia, but the doses should be matched not to achieve targets of hemoglobin, but to relieve patients suffering10 Iron deficiency should also be addressed and corrected if needed as in all CKD patients.
Selective serotonin reuptake inhibitors like fluoxetine 20mg a day or sertraline 50mg a day are good options when an anti-depressant effect is sought.
Other therapies like donepezil (a cholinesterase inhibitor used in Alzheimer´s disease), progestins or testosterone have been suggested but there are no studies proving their benefits so they should not be considered.
UREMIC PRURITUS, ITCH
Uremic pruritus is a very distressing symptom in ESRD which may contribute to worsening of other symptoms like poor sleep and depression. Its physiopathology is not wellknown.
Several pathways have been suggested: elevated histamine release by mast cells triggered by substance P associated with a systemic pro-inflammatory state, altered expression of neuro-receptors (like elevated serotonin levels) or overexpression of μ opioid receptors in dermal cells.
It has also been associated to poor control of calcium, phosphate and parathyroid hormone levels13. Xerosis (dry skin caused by sweat gland atrophy) has been suggested as an important contributing factor and a main focus of attention. General measures should be taught to patients:
– To bath in tepid water
– To use low pH shower gel and to use a soft, nonscratchy towel
– To use emollients to hydrate skin,
– To apply wet patches in scratched areas
– To avoid woolen clothes (wear light clothing)
– To pay attention to nail care
For dialysis patients, optimizing dialysis biocompatibility, efficacy and improving nutritional status has shown to improve symptoms14. All patients with uremic pruritus should be treated for hyperparathyroidism, hyperphosphatemia and hypermagnesemia to generally accepted target values15.
As in all other symptoms, secondary causes should be excluded, even if they are rare like underlying hematologic malignancies.
Topical treatments have shown good results. Capsaicin cream (with or without menthol) has been used successfully in clinical studies. Patients should be warned that it might feel like burning after application.
It should not be a reason for medication withdrawal. Ultraviolet B phototherapy has also been used with good results but requires consideration in patients with a fair complexion (skin phototypes I and II).
In most cases, the abovementioned therapies are not enough so systemic therapies must be initiated. Hydroxyzine stabilizes mast cell membranes and has sedative properties. It has been extensively used in doses of 25mg three to four times a day but it might be not tolerated because of this side effect (sedation).
Gabapentin and pregabalin have been used as alternative in doses of 100 mg and 50mg respectively after hemodialysis session (or every other day), titrated up to 300 mg or 75mg, if necessary. Its neurologic side effects (dizziness, somnolence) may be responsible for discontinuation.
Naltrexone has been used because of its effect as a μ opioid receptor agonist. It has been used in some clinical trials with positive results but its long-term safety profile has not been established. Doses from 50-100mg are appropriate but it may reverse analgesia and lead to withdrawal syndrome without major benefit.
Selective serotonin reuptake inhibitors such as paroxetine (5–10mg a day) or mirtazapine (7.5–15mg a day), a tetracyclic antidepressant, have also been used. Other immunomodulators like thalidomide or tacrolimus have not proven of any benefit, with major side effects16.
ANOREXIA
Anorexia is an important marker of uremic syndrome but it may occur as part of a broader picture: the anorexiacachexia syndrome. It is defined as a multifactorial syndrome characterized by the loss of muscle mass, associated or not with loss of adipose tissue, which cannot be reversed with conventional nutritional intake and involves loss of functional status. It must be distinguished from desnutrition that is reversible with the increase load of enteric or parenteric nutrition. It means that a nutritional assessment is mandatory17.
General measures are relevant to improve this symptom. Meals in small amounts distributed throughout the day, according to the patients tastes, is the first procedure to take. Dietitian counseling may be useful.
Food with high caloric and protein content (milk and milk products, white meat, eggs) should be favored.
Sweet flavors (butter, cheese, nuts) and cold drinks may be used to ameliorate anorexia.
As in all other symptoms, contributing factors should be corrected.
Mouth care should be encouraged. Candidiasis and xerostomia are two frequent reasons to worsen food intake. It is important to review anticholinergic drugs to reverse xerostomia.
Dysgeusia (lack of taste) is also an important factor that is very frequent in CKD patients. It may be corrected with zinc supplements.
Gastroparesis should be addressed with prokinetics like metoclopramide and constipation with laxatives.
Depression is one of most important factors which contribute to anorexia. It should be treated as usual: selective serotonin reuptake inhibitors or a tetracyclic antidepressant such as mirtazapine (7.5–15mg a day) are good options. One other important point is to evaluate the influence of social factors (Does the patient live alone? Who cooks?) and economic (Who earns money for food?). A social worker should be involved.
In ESRD patients, adequate dialysis and control of acidosis are required to avoid anorexia.
The most popular pharmacological measure is megestrol (160–400mg a day), a progestin that has been of proven benefit in over 30 studies (in non-CKD patients) with minor adverse events: (peripheral edema mainly)18.
Steroids are recommended by the European Association for Palliative Care (EAPC) in a short period to avoid side effects. Dexamethasone is the most common, in doses of 12mg/day19.
Dronabinol, a cannabinoid derivative, proved to be effective in patients with AIDS, but there are few studies in other populations and adverse effects are significant (neurotoxicity, anxiety, euphoria, drowsiness).
Nasogastric tube or parenteral feeding does not improve patient survival or comfort. In contrast, it is associated with aspiration pneumonia, sepsis, abdominal pain and diarrhea.
It is also important to explain to patients and their families that anorexia is not synonymous with malnutrition and that clinical deterioration is not due to reduced food intake but a consequence of disease progression, which is not treatable with forced intake20.
NAUSEA, VOMITING
Nausea and vomiting result from a complex antiperistaltic reflex process in response to several stimuli that culminate in activation of the chemoreceptor trigger zone in the area postrema and vomiting center in the brain stem21. The major neurotransmitters involved in the physiopathology of nausea and vomiting are dopamine, histamine, acetylcholine and serotonin. In the gastrointestinal system, the serotoninergic effect predominates, while in the vestibular system, histamine and acetylcholine are decisive.
In ESRD, nausea and vomiting are frequent symptoms (20-26%)4,5. Metabolic factors (acidosis, electrolyte disorders, uremia) are main causes of emesis but other common causes cannot be forgotten. Gastrointestinal causes like gastroesophageal reflux disease, dyspepsia diabetic gastroparesis or constipation, are too common in CKD patients. Characterization of symptom (pattern, frequency, volume), associated symptoms or comorbidities may help to indicate an etiology. Psychological, psychiatric or existential factors (anxiety, depression) must be considered. Iatrogenic factors are also very important to discontinue (opioids, steroids, digoxin, carbamazepine, iron).
Non-pharmacological measures may be more important than medication. Emetizing stimuli should be avoided. Some of these measures are indicated:
– Taking cold food (or drinks) with an appetizing appearance
– Light meals in small amounts and throughout the day
– Reduce food exposure, especially sight, odors or intense flavors (salty, greasy)
– Prefer dry foods (toast, crackers, etc.)
Reversible causes should always be addressed. It is important to keep good oral hygiene; not forget constipation (even when patients eat little), search for any hidden concerns not formulated (psychiatric, psychological or existential causes). If delayed gastric emptying is associated, food without fat should be preferred and tight clothing around the stomach avoided.
It is also essential to educate families and patients about what is happening (demystify, provide a comfortable environment, no stress at mealtimes).
Drug choice and route of administration depends on the emetogenic pathway. When a multifactorial cause is suspected, drugs with broader effects should be preferred.
Metoclopramide is a dopamine antagonist that has both antiemetic and prokinetic properties. It is effective for gastroparesis and uremia. It can be used in all routes of administration (per os, intramuscular or intravenous).
It should be started in 50% of the dose (5–10mg three times a day). Most common side effects are extrapyramidal reactions.
Low-dose haloperidol 1.5–5mg bid is an off-label option that can be used in any route of administration (oral, rectal, intravenous or subcutaneous). It is metabolized by CYP3A4 so attention must be paid to pharmacological interactions. As with the majority of dopamine antagonists, precaution should be taken with common adverse effects: urinary retention (Benign Prostatic Hyperplasia), sedation, QT prolongation. Two other drugs used in this situation because of their D2 antagonist activity are levomepromazine (6.25-25mg / day) and olanzapine (5-10mg / day).
Ondansetron, a selective 5HT3 receptor antagonist is used in doses of 4mg every 8/8hours. Mirtazapine (15mg/day), acting in the same receptor, is an alternative.
In specific cases where increased intracerebral pressure or intestinal obstruction plays an important role, dexamethasone, 4–8mg a day may be effective. Butylscopolamine (40–80mg / day) is also an option for intestinal obstruction.
The best strategy is to start one drug and if this is not effective, suspend and move to another. Starting with metoclopramide or domperidone is recommended.
Haloperidol or ondansetron are second options and, if not successful, olanzapine and lastly levomepromazine. Associations are sometimes required22.
PAIN
Pain is one of the most studied symptoms even if it is still undertreated. It is a complaint in 41% of CKD patients4. It is often associated with several etiologies and contributing factors:
– Comorbidities: diabetic neuropathy, peripheral arterial disease, pressure ulcers
– Infection: urinary tract, peritonitis, spondylodiscitis
– Renal disease: lithiasis, autosomal dominant polycystic kidney disease (ADPKD)
– Complications of renal disease: renal osteodystrophy, gouty arthropathy, calciphylaxis, amyloidosis
– Related to treatment: cramps, headache, steal syndrome, multiple vascular access punctures, abdominal pain, surgical procedures to review access
To treat pain it is essential to characterize it (acute/chronic; nociceptive /neurophatic) and to evaluate its impact (pain scales)23.
As in all other symptoms, both non-pharmacological and pharmacological procedures are important. In pain, which is perhaps the most multidimensional symptom (physical, psychological, emotional and spiritual) complementary methods used in other populations are indicated: cognitive-behavioral therapy, supportive group therapy, massages, acupuncture24.
In drug choice, the World Health Organization (WHO) analgesic ladder is appropriate, but some adaptations should be made. Non-steroid anti-inflammatories should be avoided, especially on a chronic basis because residual renal function is important to preserve. Some considerations should also be made about opioid choice. Those drugs that have renal excretion (active principle or metabolite) should be avoided because they may accumulate.
So, morphine, codeine, meperidine or oxycodone should not be used on a regular basis. Precaution should be taken when the option is for tramadol (maximum 50mg twice a day), buprenorphine (Transtec®) or hydromorphone (Jurnista®). The last two drugs have no studies to confirm their safety in CKD patients. The preference should be fentanyl, alfentanil or methadone. The basic rule for starting opioids is Go slowly, by the mouth, by the clock. Specifically in CKD patients, small doses and longer time between doses should be a priority25.
Most common side effects are constipation, xerostomia, nausea and vomiting, sedation, or sweating. Respiratory depression or opioid neurotoxicity should not be a concern if the drugs are used in small, appropriate doses.
Addition or dependence are rare in terminal illness patients so it should not be an excuse not to treat pain accordingly. Tolerance is also a rare worrying side effect that may be turned around with opioid rotation.
It is also important to remember that dialysis may remove some drugs from circulation (e.g. morphine, tramadol) so a timetable adjustment should be considered.
As recommended by the WHO analgesic ladder, adjuvants (tricyclic antidepressants, selective serotonin receptor antagonists, anticonvulsants) should be used in CKD patients if needed. Dose adjustments may be required26.
DYSPNEA
Dyspnea is a subjective experience described as difficulty of breathing or shortness of breath. It can occur in 34% of patients4 and it is one of the most worrying symptoms for patients, families and doctors.
As with pain and as it is a subjective symptom, it is multidimensional. In CKD patients, multiple modifiable causes should be considered and searched for in a careful clinical history. The most common are listed below:
– Volume overload
– Anemia
– Cardiovascular causes (e.g. cardiac insufficiency)
– Pulmonary causes (e.g. respiratory infection, COPD, pleural effusion)
– Thoracic deformities
– Increase abdominal pressure (e.g. ascites, hepatomegaly)
– Psychological / psychiatric causes (e.g. anxiety)
Optimization of volume and the prevention of distressing situations such as constipation, physical exertion, emotional stress, fever, exposure to irritant products are very important.
General measures, such as giving reassurance or simple explanations and answering the patients questions may play a very important role. The main rules are work in a peaceful and familiar environment and calm the patient: inform, educate and support to interrupt the cycle of anxiety that accompanies and worsens dyspnea. A quiet and safe place, with a family member present may make the difference.
Other non-pharmacological measures used are:
– Positioning (sitting, reclining forward to optimize vital capacity)
– Use a fan or stream cool air (stimulates trigeminal nerve)
– Respiratory kinesitherapy
– Decrease secretions
– Relaxation exercises
– Thoracentesis / Paracentesis
Specific pharmacological measures directed to the etiology should include:
– Antibiotherapy (for infection)
– Bronchodilators and steroids (for bronchospasm)
– Fluid restriction, diuretics (directed by daily weight in heart failure, besides CKD)
– Red cells transfusion (for hypoxemia)
Symptomatic treatment privileges opioids. The doses needed to control dyspnea are usually lower than those needed to control pain complaints. As stressed above, not all opioids are feasible in CKD patients. Fentanyl 12.5 μg (SC or IV every 2/2h) is the preferred option for these patients. Morphine 5mg per os should be reserved if death imminent and whenever necessary.
The second most used drugs in dyspnea are shortacting benzodiazepines (BZD) –midazolam 1.25–2.5mg, lorazepam 0.5–1mg). Their mechanism of action has been investigated beyond their effect treating associated anxiety27.
Anticholinergics, like butylscopolamine (also known as hyoscine butylbromide) 10mg every 8hours to 20mg every 4hours are important if respiratory secretions worsen the symptom. Side effects are rare and related to its anticholinergic action: xerostomia, visual disturbances, tachycardia, urinary retention, drowsiness and paradoxical agitation.
Glycopyrronium is a muscarinic anticholinergic drug frequently used to control respiratory secretions but it is not available in Portugal for parenteric administration (only in inhalor form)28.
Oxygen therapy should be reserved if hypoxemia is documented since no benefit in symptoms has been proven.
Non-invasive ventilation may be an option in patients with good physical performance, especially if cardiac insufficiency or COPD contribute to symptoms.
Last but not least, very careful attention should be paid to an optimal fluid balance, evaluated by daily weight, managed with fluid and salt restriction and diuretics. Isolated ultrafiltration may be necessary, particularly in an acute setting.
ANXIETY AND DEPRESSION
Anxiety is a common symptom when patients face the uncertainty of disease and particularly when death approaches. When anxiety becomes disproportional there are several aspects that should be evaluated. Once more, biological, psychological, social and spiritual factors contribute to this symptom. It is important to know the patient to manage it: listen to the patient, explore his or her fears. It is crucial to analyze all stressors and to communicate with patient and family. The patient should feel that control of his or her life is reestablished. When associated to depression, it may be difficult to evaluate the sadness of someone who has a life-threatening disease.
Although depression should not be trivialized or neglected. In a patient on dialysis it is mandatory to exclude depression when he ask to stop dialysis. It is important to identify predisposing factors because they may be the target of non-pharmacological therapies. Psychotherapy may be required. A psychologist may be useful to find coping strategies.
Frequently pharmacological measures can help. Short-acting benzodiazepines (lorazepam 1mg, oxazepan 15mg, alprazolam 0.25mg) are indicated. In cases of chronic anxiety, long term BZD may be used (e.g. clonazepam 0.5mg). Most common side effects are drowsiness, fatigue, difficulty concentrating and hypotonia. They are not usually serious enough to stop medication.
Buspirone is a good option in chronic anxiety or adaptation disorders but with delayed onset action. It has a low profile of side effects.
Haloperidol may be used when BZD are not enough to control the symptom. It presents benefits when anxiety is accompanied by other organic symptoms like nausea and vomiting.
Trazodone 100mg/day is a tricyclic antidepressant with anxiolytic effect that may be an option, especially if there are sleep disturbances because of its sedative effect with less anticholinergic action.
Escitalopram 10mg/day is an antidepressant with selective serotonin re-uptake properties indicated when anxiety is associated. Side effects profile is similar to other serotoninergic agents.
Amitriptyline 25mg/day (a tricyclic antidepressant) may be used when pain is a worrying symptom. Side effects are anticholinergic ones, particularly hypotension and cardiac rhythm alteration29.
CONCLUSION
Symptom burden is high in ESRD patients but it is still under-recognized and under-treated. Symptom assessment should be part of a routine review of CKD patients.
Nephrologists should be familiar with palliative tools to better control symptoms, in addition to the usual care.
Most symptoms management strategies are extrapolated from other palliative populations, especially cancer ones. Team-working between nephrologists and palliative medicine professionals is essential to allow optimum management of the patient. Further research is required and more studies are needed to determine how pharmacology is affected by renal failure and which specificities occur in CKD patients.
References
1. Saini T, Murtagh FE, Dupont PJ, McKinnon PM, Hatfield P, Saunders Y. Comparative pilot study of symptoms and quality of life in cancer patients and patients with end stage renal disease. Palliat Med. 2006;20(6):631-636 [ Links ]
2. Murphy EL, Murtagh FE, Careu I, Sheerin NS. Understanding symptoms in patients with advanced chronic kidney diseasemanaged without dialysis: use of a short patientcompleted assessment tool. Nephron Clin Pract. 2009;111(1):c74-c80 [ Links ]
3. Davison SN, Jhangri GS, Johnson JA. Longitudinal validation of a Modified Edmonton Symptom Assessment (ESAS) in Haemodialysis patients. Nephrol Dial Transplant. 2006;21(11):3189-3195 [ Links ]
4. Yong DS, Kwok AO, Wong DM, Suen MH, Chen WT, Tse DM. Symptom burden and quality of life in end-stage renal disease: a study of 179 patients on dialysis and palliative care. Palliat Med. 2009;23(2):111-119 [ Links ]
5. Weisbord SD, Fried LF, Arnold RM, Fine MJ, Levenson DJ, Peterson RA, Switzer GE. Prevalence, severity and importance of physical and emotional symptoms in chronic hemodialysis patients. J Am Soc Nephrol. 2005;16:2487-2494 [ Links ]
6. Abdel-Kader K, Unruh ML, Weisbord SD. Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin J Am Soc Nephrol. 2009;4(6):1057-1064 [ Links ]
7. Jablonski A. Level of symptom relief and the need for palliative care in the hemodialysis population. JHPN. 2007;9(1):50-58 [ Links ]
8. Murtagh FE, Addington-Hall JM, Edmonds PM, Donohoe P, Carey I, Jenkins K, et al. Symptoms in advanced renal disease: a cross-sectional survey of symptom prevalence in stage 5 chronic kidney disease managed without dialysis. J Palliat Med. 2007;10(6):1266-1276 [ Links ]
9. Murtagh FE, Addington-Hall J, Higginson IJ. The prevalence of symptoms in end-stage renal disease: a systematic review. Adv Chronic Kidney Dis. 2007;14(1):82-99 [ Links ]
10. Radbruch L, Strasser F, Elsner F, Gonçalves JF, Løge J, Kaasa S, et al. Fatigue in palliative care patients – an EAPC approach. Palliat Med. 2008;22(1):13-32 [ Links ]
11. Yennunjalingam S, Bruera E. Palliative management of fatigue at the close of life. JAMA. 298(2):295-304 [ Links ]
12. Barbosa A, Pina PR, Tavares F, Neto IG. Manual de Cuidados Paliativos. 3rd Edition. Faculdade de Medicina de Lisboa, 2016:215 [ Links ]
13. Lugon JR. Uremic pruritus: a review. Hemod International. 2005;9:180-188 [ Links ]
14. Hiroshige K, Kabashima N, Takasugi M, Kuroiwa A. Optimal dialysis improves uremic. Am J Kidney Dis. 1995;25(3):413 [ Links ]
15. Chou FF, Ho JC, Huang SC, Sheen-Chen. A study on pruritus after parathyroidectomy for secondary hyperparathyroidism. J Am Coll Surg. 2000;190(1):65
16. Kobrin SM. Uremic pruritus. Up to date. Accessed April 2017 [ Links ]
17. Fearon K, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;32:489-95 [ Links ]
18. Berenstein EG, Ortiz Z. Megestrol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst Rev. 2005;CD004310 [ Links ]
19. Radbruch L, Elsner F, Trottenberg P, Strasser F, Fearon K. Clinical practice guidelines on cancer cachexia in advanced cancer patients. European Palliative Care Research Collaborative. 2010 [ Links ]
20. Alves M, Abril R, Neto IG. Symptomatic control in end-of-life patients. Acta Med Port. 2017;30(1):61-68 [ Links ]
21. Haris DG. Nausea and vomiting in advanced cancer. Br Med Bull. 2010;96:175-185 [ Links ]
22. Barbosa A, Pina PR, Tavares F, Neto IG. Manual de Cuidados Paliativos. 3rd Edition. Faculdade de Medicina de Lisboa, 2016:101-119 [ Links ]
23. Pham PCT, Toscano E, Pham PMT, Pham PAT, Pham SV, Pham PTT. Pain management in patients with chronic kidney disease. NDT Plus. 2009;2:11-118 [ Links ]
24. Kafkia T, Chamney M, Drinkwater A, Pegoraro M, Sedgewick J. Pain in chronic kidney disease: prevalence, cause and management. J Ren Care. 2011;37(2):114-122 [ Links ] Davison SN, Ferro CJ. Management of pain in chronic kidney disease. Progr Palliat Care. 2009;17(4):186-195 [ Links ]
26. Davison SN, Koncicki H, Brennan F. Pain in chronic kidney disease: a scoping review. Semin Dial. 2014;27(2):188-204 [ Links ]
27. Jenkins K, Bennett L, Ho TM. Conservative Management in Advanced Kidney Disease. A guide to Clinical Practice. EDTNA/ERCA. Sept 2011;138-140 [ Links ]
28. Douglas C, Murtagh FEM, Chambers EJ, Howse M, Ellershaw J. Symptom management for the adult dying with Advanced Chronic Kidney Disease: a review of the literature and development of evidence-based guidelines by United Kingdom Expert Consensus Group. Palliat Med. 2009;23:103-110 [ Links ]
29. Porta J, Batiste XG, Tuca A. Manual de control de síntomas en paciente con câncer avanzado y terminal. 2nd edition. Aran Editor, 2008:160-169 [ Links ]
Ana Farinha
Nephrology Department, Centro Hospitalar de Setúbal, Portugal
Rua Camilo Castelo Branco, 2910-446Setúbal
E-mail: alpfarinha@gmail.com
Disclosure of potential conflicts of interest: none declared.
Received for publication: May 4, 2017
Accepted in revised form: Sep 7, 2017














