Serviços Personalizados
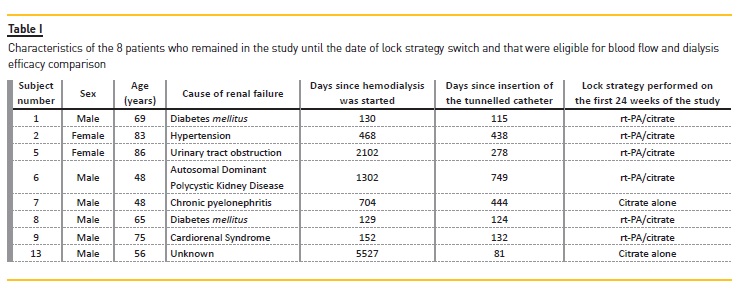
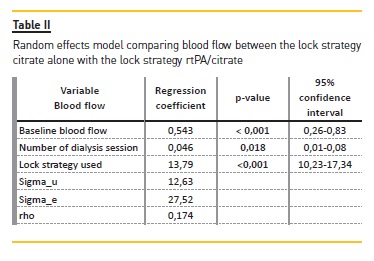
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.31 no.2 Lisboa jun. 2017
REVIEW ARTICLE
Are there benefits of a high potassium diet, even in the CKD patient?
Biff F. Palmer1, Deborah J. Clegg2
1 Professor of Internal Medicine, Department of Internal Medicine, University of Texas Southwestern Medical Center, Dallas, Texas
2 Professor of Internal Medicine, Biomedical Research Department, Diabetes and Obesity Research Institute, Cedars-Sinai Medical Center, Beverly Hills, California
ABSTRACT
Recent data have demonstrated dietary intake of potassium (K+) is well below current recommended nutritional guidelines, and K+ deficiency has been implicated in many diseases to include cardiovascular disease, kidney stones, and osteoporosis. Importantly, dietary supplementation of K+ has favorable effects in reducing blood pressure, decreasing the risk of stroke, improving bone health, and reducing the risk of nephrolithiasis. Unfortunately, the combination of westernized societies where consumption of foods enriched in K+ such as fresh fruits and vegetables is low, with the advent of widely prescribed hypertensive medications which cause hyperkalemia thereby requiring dietary K+ restriction, have led to societal concerns of low K+ consumption leading to decrements in overall public health. Importantly, there are new and novel drugs which have been developed which are capable of binding K+ in the gastrointestinal tract, allowing for diet liberalization affording patients the numerous health benefits of K+ rich diets. Here we highlight new findings indicating there are health‑related benefits of K+ consumption even in the patient with reduced renal function such as the chronic kidney disease patient. Lastly we provide recommendations to include the use of new K+ binding agents which can allow liberalization of diets in patients with impaired renal function without development of hyperkalemia.
INTRODUCTION
Potassium (K+) is an extremely important mineral, and the recent Dietary Guidelines for Americans (DGAC, 2016) designated potassium as a nutrient of public health concern due to its general under consumption.
This lack of adequate K+ intake has been associated with the increased prevalence of hypertension and cardiovascular diseases, two common adverse diet‑related health outcomes. Potassium is now listed as a nutrient of public health significance due to recent data where it has been estimated mean intake of K+ to be 2,290 mg/day for women and 3,026 mg/day for men, substantially lower than the recommended values.1
Western industrialized societies also suffer from diets which are high in sodium (Na+), resulting in daily intake of sodium chloride to be about three times higher than the daily intake of K+ on a molar basis. The changes in the amount of K+ and Na+ intake over time reflect societal shifts from traditional plant based diets enriched in K+ content and low in Na+ (characterized by fruits, leafy greens, roots, and tubers), to processed foods high in Na+ and low in K+. The transition to processed foods began approximately 10,000 years ago with the onset of agriculture. Perhaps inadequate K+ consumption in the presence of excessive amounts of sodium sets up a mismatch contributing to the pathophysiology of a variety of chronic diseases such as obesity, hypertension, diabetes, kidney stones, and bone disease.
The normal kidney has the capacity to maintain K+ homeostasis in the setting of high dietary intake, as demonstrated by the fact that serum K+ levels are maintained in the normal range even when dietary K+ intake is increased to approximately 15 gms daily for 20 days.2,3 This ability to maintain normal serum K+ concentrations when challenged with large K+ loads over a prolonged period, suggests humans are able to prodigiously defend K+ homeostasis (Table 1). Mechanisms by which K+ homeostasis is maintained are discussed below.
OVERVIEW OF RENAL K+ HANDLING
K+ is freely filtered by the glomerulus followed by reabsorption in the proximal tubule and thick ascending limb, with only small amounts reaching the distal nephron. Reabsorption of K+ in the proximal tubule is primarily through the paracellular pathway, and this is roughly in proportion to Na+ and water. The apical membrane Na+‑K+ ‑2Cl‑cotransporter mediates transcellular K+ transport in the thick ascending limb of Henle. K+ secretion begins in the early distal convoluted tubule (DCT), and the magnitude progressively increases into the cortical collecting duct. As recently reviewed, the secretory component of K+ handling is that which varies and is regulated according to physiologic needs.4
The major K+‑secretory mechanism in the distal nephron is electrogenic secretion through the ROMK (renal outer medullary K+) channel. A second channel (maxi‑K+ or BK channel) also mediates K+ secretion under conditions of increased flow.5 In addition to stimulating maxi‑K+ channels, tubular flow also augments electrogenic K+ secretion by diluting luminal K+ concentration and stimulating Na+ reabsorption through the epithelial Na+ channel (ENaC).
In part, this stimulatory effect can be traced to a mechanosensitive property whereby shear stress increases the open probability of the ENaC channel.6
Na+ and K+ transport in the distal nephron buffer any increase in extracellular K+ concentration following a K+ rich diet. High dietary K+ content leads to an increase in glomerular filtration rate and tubular flow, and the increased flow through the distal nephron increases distal Na+ delivery and dilutes luminal K+ concentration both of which augment electrogenic K+ secretion through the ROMK channel. Along with flow mediated activation of maxi‑K+ channels, these events enhance renal K+ secretion thus providing a defense against development of hyperkalemia.
Additionally, there is an increased medullary recycling and accumulation of K+ in the interstitium of the kidney, which decreases Na+ reabsorption both in the thick ascending limb and proximal tubule further providing a mechanism for high K+ intake to increase tubular flow.7‑10
More recent studies have focused on how K+ intake modulates transport in the low capacity early DCT as a way to adjust tubular flow to K+ secretory sites. Additionally, K+‑rich foods, such as fruit and vegetables, are also rich in precursors to bicarbonate ions.
The alkali present in such a diet directly effects the determinants of K+ transport in the DCT so as to facilitate the renal excretion of the co‑ingested K+ load.11,12
CLINICAL BENEFIT OF K+ SUPPLEMENTATION
Hypertension
Epidemiologic studies have established K+ intake is inversely related to the prevalence of hypertension13; specifically, K+ supplements have been shown to lower blood pressure in hypertensive subjects. This is in contrast to findings indicating blood pressure increases in hypertensive subjects placed on a low K+ diet. Elevations in blood pressure in the presence of a low K+ diet are associated with increased renal Na+ reabsorption.14 In further support of this, data generated from an NHANES III survey of 17,000 individuals suggested elevated dietary K+ intakes were associated with significantly lower blood pressures.15 An additional study called Dietary Approaches to Stop Hypertension (DASH) trial also found beneficial blood pressure effects of a K+‑rich diet.16 The study compared diets which consisted of 3.5 servings/day of fruit and vegetables and 1,700 mg/day of K+ to the DASH diet which included 8.5 servings/day of fruit and vegetables and 4,100 mg/day of K+. These findings specifically indicated the high K+ diet was associated with lowering blood pressure by an average of 2.8/1.1 mm Hg in all subjects, and by an even greater amount of 7.2/2.8 mm Hg in those with individuals with hypertension.
Additionally, enhancing K+ intake to 2,300‑3,900 mg/day significantly reduced blood pressure by an average of 1.8/1.0 mm Hg in people with normal blood pressure and 4.4/2.5 mm Hg in people with hypertension.17
These blood pressure lowering effects were influenced by race, and were more pronounced in black individuals, and those who consumed higher amounts of Na+.18
Renal conservation of K+ and Na+ under conditions of dietary K+ deficiency may have evolved because typically dietary K+ and Na+ deficiency likely occurred together; however, this adaptation is now deleterious due to the westernization of our diets which are low in K+ and high Na+. Additionally, low K+ intake may contribute to increased sympathetic tone as a mechanism causing hypertension. As recently reviewed, Na+ excess and K+ deficiency can alter the electrolyte and hormonal composition of the cerebral spinal fluid, activating sensors which through the hypothalamus lead to neurohumoral activation.19
Stroke
Epidemiological studies have suggested increased K+ intake is associated with reductions in risk of stroke.
43,000 men followed for eight years in a prospective study found those with the highest dietary K+ intake (median intake, 4,300 mg/day) were significantly less likely (62%) to have a stroke when compared to those with the lowest K+ intake (median intake, 2,400 mg/day).20 In an additional analysis of 11 cohort studies with a total of 127,038 participants, K+ intake in the range of 90‑120 mmol/day was associated with reductions in risk of stroke (RR 0.79, 95% confidence interval 0.68 to 0.93).18 In another study, they found that low dietary K+ intakes (<34.6 mmol/d) in 9805 men and women followed over an average of 19 years was associated with a 28% higher hazard of stroke.21 Lastly in another study, 90,137 postmenopausal women aged 50 to 79 followed prospectively for an average of 11 years, the investigators found those with the higher K+ intake had a lower risk for strokes.22 Taken together, these strong epidemiological data suggest modest K+ increases, especially in hypertensive individuals, significantly reduces the risk of stroke.23
Osteoporosis
K+‑rich foods, such as fruits and vegetables, are rich in precursors to bicarbonate ions, which buffer acids in the body. As previously mentioned, the Western diet is low with respect to sources of alkali‑enriched foods (fruit and vegetables) and high in sources of acid (fish, meats, and cheeses). When the quantity of bicarbonate ions is insufficient to maintain normal pH, the body mobilizes alkaline calcium salts from the bone in order to neutralize the acids consumed in the diet. The net acid content of the diet is reduced following consumption of fruit and vegetables which may preserve calcium in bones. Additionally, an alkaline diet which is high in K+ may exert an anion‑independent effect on bone metabolism. In a study of 266 elderly postmenopausal women, increased dietary K+ was associated with significantly higher hip (at 1 and 5 years) and total body (at 5 years) bone mineral densities when compared to those individuals with lower K+ intakes.24 Higher consumptions of fruits and vegetables have also been associated with improvements in bone mineral density as well as biomarkers of bone formation in premenopausal, postmenopausal women, and elderly men.25
Nephrolithiasis
Development of kidney stones is associated with abnormally high urinary calcium levels (hypercalciuria) and decreased urinary citrate. In individuals with a history of developing calcium‑containing kidney stones, intake of diets high in acid are significantly associated with increased urinary calcium excretion and decreased urinary citrate. Importantly, reductions in K+ intake have been found to increase urinary calcium excretion as well as reduce urinary citrate.26,27; therefore, increasing dietary K+ (and thereby increasing the alkali content) has been found to reverse these effects. These data demonstrate consumption of K+‑rich foods significantly reduces the risk of kidney stones.28,29
USE OF POTASSIUM ENRICHED DIET IN CHRONIC KIDNEY DISEASE
Consumption of K+‑enriched diets provides all of the benefits discussed above, but it is important to note that much of this information has been determined from studies in individuals with normal renal function.
Do the same diets provide benefit to those with impaired renal function? Development of life threatening hyperkalemia has limited the ability to utilize K+‑enriched diets in patients with impaired renal function, such as those with chronic kidney disease. The presence or the occurrence of hyperkalemia in these individuals provides a therapeutic dilemma since diets enriched in fruits and vegetables may actually offer therapeutic benefits unique to the chronic kidney disease patient. These benefits are discussed below.
Phosphorus control
Nutritional management in patients with chronic kidney disease focuses on maintenance of adequate protein intake and prevention of phosphate overload and hyperphosphatemia. The phosphorus in foods from plant proteins has a lower absorption rate than phosphorus from animal proteins. Specifically, phosphorus in plant sources is about 40 to 50% absorbed because plant phosphorus is in the form of phytates and mammals lack enzymes called phytases which facilitate break down and absorption. This is in contrast to the phosphorus in animal proteins, which is readily hydrolyzed and absorbed.30 In individuals with chronic kidney disease, phosphate homeostasis was examined in a crossover trial of nine patients by comparing a diet enriched with animal or vegetable protein for seven days.31 Despite an equivalent amount of protein and phosphorus concentration in both diets, ingestion of the vegetarian diet resulted in lower serum phosphorus levels consistent with reduced gastrointestinal absorption of phosphate.31 These data suggest consumptions of diets enriched in plant proteins in chronic kidney disease patients may facilitate intake of adequate protein levels with reduced incidence of hyperphosphatemia.
Control of Metabolic Acidosis
Chronic metabolic acidosis is due to a reduction in renal mass often prevalent in the chronic kidney disease patient which leads to reductions in the capacity for net acid excretion. In this patient population, metabolic acidosis treatment is a goal. Maintenance of the serum bicarbonate concentrations in the normal range (23 to 29 mEq/L) may avoid complications of chronic acidosis to include protein‑energy wasting, insulin resistance, bone demineralization and progression of renal disease.32,33 This is normally achieved therapeutically by providing oral supplementation of NaHCO3; however, this treatment is associated with increased sodium intake and can exacerbate the volume expansion and hypertension commonly present in chronic kidney disease.
This management is not well tolerated by patients, and an alternative approach would be to increase the consumption of fruits and vegetables thereby providing a source of alkali precursors which is not accompanied by a salt load.34,35 This strategy was tested in a group of patients with stage 2 chronic kidney disease due to hypertensive nephrosclerosis. Urine indices of renal injury were measured and compared after 30 days of increased fruit and vegetable consumption versus oral
NaHCO3 therapy. Reduction in urinary albumin and N‑acetyl β‑D‑glucosaminidase were similar between the 2 groups. Fruit and vegetable consumption (which is enriched in K+ as discussed previously) was associated with a significantly greater reduction in systolic blood pressure.36 In a similar study, 71 patients with stage 4 CKD and serum bicarbonate <22 mEq/L were randomized to 1 year of sodium bicarbonate at 1.0 mEq/kg per day or increased fruits and vegetables to reduce dietary acid by half.37 The serum bicarbonate increased with the dietary intervention, although less than what occurred in bicarbonate group, whose alkali dose would be expected to nearly completely neutralize the dietary acid load. The aforementioned markers of kidney injury declined similarly in the 2 groups. These findings suggest the alkali load afforded by a diet enriched in fruits and vegetables can slow the progression of chronic kidney disease through correction of metabolic acidosis.
In addition to avoiding the salt load and potential volume overload that can complicate NaHCO3 therapy, the high K+ diet is associated with reductions in blood pressure possibly related to increased K+ intake. It is important to note that there were no complications from hyperkalemia, but only patients with plasma K+≤4.6 mEq/L were enrolled in the study.
PHARMACOLOGY USED TO REDUCE THE RISK OF HYPERKALEMIA:
Despite the benefits of a K+ enriched diet, there is a subset of individuals who are at risk for hyperkalemia.
The initial approach to managing their hyperkalemia is eliminating other sources of K+ such as supplements (to include those found in herbal medications), discontinuing prescribed or over‑the‑counter drugs known to interfere in renal K+ excretion (nonsteroidal anti‑inflammatory drugs), and ensure effective diuretic therapy.38 Correction of metabolic acidosis is also useful although this may be at least partially addressed by the alkali load found in the fruits and vegetables ingested.
Management of hyperkalemia has relied on chronic use of sodium polystyrene sulfonate (Kayexalate), which binds K+ in the gastrointestinal tract; however, this is poorly tolerated and has been linked to gastrointestinal toxicity. Recently, there is new and novel pharmacology, Patiromer and sodium zirconium cyclosilicate, which are K+ binding drugs shown to be effective in preventing development of hyperkalemia. Patiromer (Veltassa), a recently FDA‑approved drug, is a nonabsorbed polymer that binds K+ in the gastrointestinal tract, predominately in the colon. Patiromer effectively decreases serum K+ concentrations in high‑risk patients on RAAS blockers, including those with heart failure, chronic kidney disease, and diabetic nephropathy.39 In a study of over 300 patients with diabetic nephropathy with either mild to moderate hyperkalemia, the drug lowered serum K+ concentrations in a dose dependent manner with the greatest reduction in those with higher starting values. The drug remained effective in controlling plasma K+ concentrations over a 44‑week maintenance phase despite ongoing administration of RAAS inhibitors.40 The drug was well tolerated with the main adverse events being constipation and hypomagnesemia, which required magnesium replacement in a small number of subjects.
Sodium zirconium cyclosilicate is a non‑absorbed microporous compound which binds K+ throughout the gastrointestinal tract. The pore size renders it highly selective for the K+ ion as compared to calcium or magnesium ions. Like patiromer, this drug has also been shown effective in lowering plasma K+ concentration in a dose depend manner with the greater reductions in those with the highest levels.41,42 However, despite being well tolerated, there are reports of edema at higher doses.
It is important to note that dietary K+ intake was not specifically controlled for in the clinical trials with patiromer and sodium zirconium cyclosilicate. While the effectiveness of these drugs in patients purposely ingesting a K+ enriched diet has not been studied, their ability to control plasma K+ concentration in those subjects with a history of hyperkalemia despite ongoing use of RAAS blockers suggests these agents could prove useful in allowing patients at risk for hyperkalemia to liberalize their diets to be enriched in fruits and vegetables.
In addition to improving the quality of life through liberalization of diet, these high risk patients could enjoy the cardiovascular and metabolic benefits affordedby such a dietary change.
IMPLEMENTATION OF K+ ENRICHED DIETS IN PATIENTS AT RISK FOR HYPERKALEMIA
There are a wealth of data suggesting diets rich in sources of K+ reduce blood pressure, reduce the risk of stroke, improve bone health, control metabolic acidosis, and reduce phosphorus accretion, and such diets can be prescribed in patients with normal renal function with little risk of complications. By contrast, a K+ enriched diet given to a patient with impaired renal function increases the risk of developing hyperkalemia.
There are no long term studies examining the benefits of a K+ enriched diet in patients with chronic kidney disease because of the concern for developing life threatening hyperkalemia. In fact, management of early declines in renal function often include some degree of K+ restriction in the diet particularly those being treated with a renin‑angiotensin‑aldosterone (RAAS) blocker. Aside from the potential benefits of better phosphate control and amelioration of metabolic acidosis discussed previously, the question arises if liberalization of dietary K+ in patients with impaired renal function may provide long term cardiovascular benefits.
Additionally, K+ restriction is further reinforced as patients transition to end stage renal disease and ultimately dialysis. With the advent of novel K+ binding agents, it is now interesting to speculate if clinical trials focusing on liberalization of the diet to include sources of K+ enriched foods, may provide some cardiovascular benefits in these patients who might most benefit.
Although not specifically tested as an approach to prevent diet induced hyperkalemia, these new agents are well tolerated and could be utilized to study whether patients with impaired renal function would benefit from such a diet. At a minimum, these agents could facilitate a relaxation in dietary K+ restriction potentially contributing to a better quality of life.
CONCLUSION
There is an abundance of data suggesting consumption of diets high in K+ are beneficial and may reduce the incidence of stroke, hypertension, nephrolithiasis, and osteoporosis. The kidney is exquisitely designed to maintain K+ homeostasis even in the presence of high K+ intakes. Westernized diets which are high in processed foods, high in Na+ content, and low in K+, are associated with significant morbidity and mortality.
Despite this, our first line of treatment in patients with reduced renal function is to place them on low K+ diets.
One could argue that these individuals would benefit most from increasing their intake of K+ rich foods due to the numerous health benefits associated with fruit and vegetable consumption. Currently, the standard treatment for hypertension is the use of RAAS blockers; however, a limiting factor in their use is development of hyperkalemia necessitating either reductions in therapeutic dosing or placing these individuals on a low K+ diet. With two new therapeutic options to chronically treat hyperkalemia, there is an opportunity to advocate for dietary liberalization of K+ even in the setting of ongoing RAAS blockade to maximize cardiovascular benefit. It is an important time to re‑think the role of K+ in the diet. Reducing consumption of processed foods and increasing consumption of fruits and vegetables is associated with improvements in health, and here we highlighted that K+, an often under‑appreciated cation/mineral, may be directly related to these benefits.
References [ Links ] Rabelink TJ, Koomans HA, Hene RJ, Dorhout Mees EJ. Early and late adjustment to potassium loading in humans. Kidney Intl 1990;38:942‑7. [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] Sufit CR, Jamison RL: Effect of acute potassium load on reabsorption in Henles loop in the rat. Am J Physiol 245: F569‑76,1983 [ Links ] Brandis M, Keyes J, Windhager EE. Potassium‑induced inhibition of proximal tubular fluid reabsorption in rats. Am J Physiol 1972;222:421‑7. [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] Krishna GG, Kapoor SC: Potassium depletion exacerbates essential hypertension. Ann Intern Med 115: 77‑83, 1991 [ Links ] Hajjar IM, Grim CE, George V, Kotchen TA. Impact of diet on blood pressure and age‑related changes in blood pressure in the US population: analysis of NHANES III. Arch Intern Med 2001;161(4):589‑593. [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ] [ Links ]
Deborah J. Clegg, Ph.D
Professor Biomedical Research Department Diabetes and Obesity Research Division Cedars‑Sinai
Medical Center
Beverly Hills, California
310‑967‑2787 (w)
E-mail: Deborah.clegg@cshs.org
Disclosure of potential conflicts of interest: none declared
Received for publication: May 8, 2016
Accepted in revised form: May 12, 2017














