Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Portuguese Journal of Nephrology & Hypertension
Print version ISSN 0872-0169
Port J Nephrol Hypert vol.31 no.1 Lisboa Mar. 2017
CASE REPORT
Kidney transplantation in patients with preformed and exclusively anti-HLA-Cw donor specific antibody
Sofia Santos1, Ana Castro1, Andreia Campos1, Sofia Pedroso1, Leonídio Dias1, Castro Henriques1
Serviço de Nefrologia e Transplantação Renal, Centro Hospitalar do Porto, Porto, PORTUGAL.
ABSTRACT
We report a patient who had received a first kidney transplant and had preformed DSA anti-HLA-Cw, developing AMR C4d+ soon after transplant. Classically anti-HLA-Cw are considered less immunogenic and are not considered in many organ allocation systems or immunologic risk stratification algorithms, including in Portugal. However, data from literature confirms that their presence is as deleterious as DSA anti-HLA A/B/DR/DQ. Thus we should take HLA-C typing and respective antibody identification into account in sensitized patients, in order to access risk stratification and establish the need for correct induction or desensitization therapies.
Key-Words: Donor-specific antibodies (DSA), anti-HLA-Cw antibodies, antibody-mediated rejection (AMR).
INTRODUCTION
Forty to 50 percent of renal transplant sensitized recipients may have preformed anti-HLA-Cw antibodies.
However, this frequency is lower than the reported 70 to 80% for the other classical major human leukocyte antigen (HLA) Class I (A and B)1,2.
For many years anti-HLA-Cw antibodies were considered less immunogenic3 and were not considered in many organ allocation systems or immunologic risk stratification algorithms, including in Portugal.
More recently, with the development of more sensitive assays to detect HLA antibodies, namely solidphase immunoassays, some clinical reports and retrospective studies have shown that HLA-C locus induces an antibody-mediated response similar to the other routinely checked loci2,4-7. The existence of preformed anti-HLA donor-specific antibodies (DSA) prior to transplant increases the risk of antibody-mediated rejection (AMR)8 and is a factor contributing to poorer graft survival9. To address and highlight the importance of this subject we present a patient who had received a first kidney transplant and had preformed DSA anti-HLA-Cw, developing AMR C4d+ soon after transplant.
CASE REPORT
A 50-year-old caucasian female with end-stage renal disease due to polycystic kidney disease received a deceased kidney allograft transplant at our centre. She had started dialysis 3 years and 11 months prior. Regarding possible sensitization events she had no history of previous organ transplantation or transfusions but had had 3 previous pregnancies. She underwent right nephrectomy 3 years earlier due to small abdominal cavity hampering implantation of a donor kidney.
The donor was a 56-year-old male with subarachnoid haemorrhage as cause of death. The recipient and the donor were both CMV-positive. The ABO blood type of the donor and recipient was compatible, and the HLA alleles were mismatche at five loci: A1, B51, B62, DR4 and DR13 (Table I).
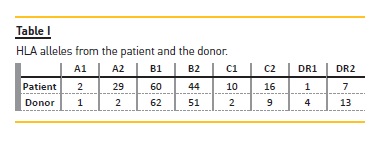
The crossmatch was negative in complementdependent cytotoxicity, negative in flow cytometry for B-cells (B-cell channel shift: 38, with cut-offs set at 80), but positive in flow cytometry for T-cells (B-cell channel shift: 69, with cut-offs set at 50). Cytotoxic PRA both in peak and last serum before transplant was 0%.
To determinate the specificity of the HLA antibodies, a single-antigen bead (SAB) assay (LabScreen Single Antigen Beads®, OneLambda, Canoga Park, CA) was performed in the patient. The MFI of each bead was measured using LABScanTM 100 flow analyzer (Luminex®, Austin, TX, USA). The analysis was performed using HLA fusion® software (One Lambda Inc.) and a cut-off for a positive reaction set at MFI value of ≥1000.
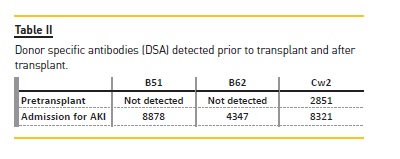
Several anti-HLA antibodies were identified: B13-8217, Cw6-4445, Cw18-4086, Cw15-3961, Cw17-3466, Cw5-3306, Cw2-2851, Cw4-1621, but only the Cw2 was donor-specific.
Immunosuppressive treatment included antithymocyte globulin (3 mg/kg for 7 days), tacrolimus (FK-506, 0.1 mg/kg/day, and the dose was adjusted to maintain a trough level in whole blood between 8 and 12 ng/ml during the first month), mycophenolate mofetil (started at a dose of 2000 mg/day) and corticosteroids (methylprednisolone was administered intravenously at doses of 500, 250 and 125 mg/day on the day of transplantation, days 1-2 and days 3-4 after the operation, respectively; oral prednisolone was started on day 5 after the operation at the dose of 20 mg, then tapered to 15 mg/day within first month after transplant).
Surgery went well, with no reported intraoperative complications. Ureterocystostomy was protected by a JJ-stent. The patient started diuresis immediately postoperative.
Low blood pressure and a haemoglobin fall of 3grams/dL were detected in the hours following surgery, but there was no active bleeding identified by ultrasound.
The patient responded to fluids and 2 units of red blood cells. On the subsequent days, the allograft evolved with good early function and the patient was discharged after 8 days with serum creatinine level of 1.01 mg/dL.
Three days after discharge, she presented asymptomatic to her routine first transplant consultation but with de novo increased serum creatinine (level 2.93 mg/dL) and urea of 198 mg/dL. Tacrolimus levels were within the range. Renal sonography revealed good contrast resolution between cortex and medulla, no signs of dilatation of the collecting system, with pig-tail catheter correctly positioned. She was admitted and a renal allograft biopsy was performed.
The biopsy findings were highly indicative of acute AMR with glomerulitis (g2), peri-tubular capillaritis (ptc2), diffuse acute tubular injury and linear diffuse positive C4d staining at peritubular capillaries.
Repeated testing using Luminex® SAB assay was performed (for Class I and Class II antigens) on the serum samples from day 1 of the current admission.
We repeated the crossmatch and this time it was positive in flow cytometry for B-cells (B-cell channel shift: 69, with cut-offs set at 80), and positive in flow cytometry for T-cells (B-cell channel shift: 143, with cut-offs set at 50).
Three DSA were detected against HLA-B51 (with a MFI of 8878), against HLA-B62 (with a MFI of 4374) and again against HLA-Cw2 (with an increase of MFI from 2851 to 8321) – Table II. The C1q fixation capacity of anti-HLA antibodies by Luminex C1q assay was not performed.
The clinical and histological findings were consistent with an acute AMR, as established by the Banff criteria10.
She was treated with methylprednisolone (500 mg bolus) for 3 days, plasmapheresis (7 sessions every other day) in combination with intravenous immunoglobulin (IvIg) 100 mg/kg after each session. After the last plasmapheresis session, she received high-dose IvIg (2 g/kg) divided into four daily doses, followed by one dose of rituximab (375 mg/m2); a similar dose of IvIg (2 g/kg) was repeated 1 and 3 months later.
Six months after, her graft function was recovered to serum creatinine 1.1 mg/dL without proteinuria.
DISCUSSION
Preformed human leukocyte antigen (HLA) donor specific antibodies (DSA) have been shown to increase the risk of antibody-mediated rejection (AMR) and have a deleterious effect on kidney graft survival11,12. Classically, antibodies to major HLA Class I (A and B) and Class II (DR and DQ) are considered to be responsible for the majority of the cases of AMR. For many years HLA-Cw antigens were considered less immunogenic and neglected in the matching algorithms of most kidney allocation systems. HLA-Cw molecules are poorly expressed at the cell surface compared with HLA-A and HLA-B locus products, but intracellular HLA-A, HLA-B and HLA-Cw alleles are expressed in similar amounts13,14.
One reason suggested for this low expression at the cell surface is the fact that HLA-Cw alleles interact stably with the transporter associated with antigen processing (TAP) and they are retained in the endoplasmic reticulum, where they are degraded13. An additional explanation for the poor HLA-Cw cell surface expression, suggested by McCutcheon et al.14, is that HLA-Cw heavy chain mRNA is instable and rapidly degraded, resulting in a lower rate of protein. Those facts, in association with the modest sensitivity of the lymphocytotoxicitybased assays used for screening and identification of HLA-Cw antigens are the main reason for their disregard.
In spite of their frequency being lower, with reports of around 50% sensitization to HLA-Cw, and around 80% to HLA-A and HLA-B1,2, some reports have been published concerning their association with AMR and impact on graft function and survival5,15,16.
Gilbert et al. compared two groups of pretransplant immunized recipients, one with only classical HLA-A, -B, -DR, -DQ antibodies (n = 176) and the other group with classical plus HLA-C and/or -DP antibodies (n = 27)4. They concluded that there was a significant increase in the number of AMR and graft losses for immunologic reasons among the group with pretransplant anti-Cw and -DP antibodies. However, they did not distinguish between pretransplant anti-DP or anti-Cw antibodies, and they speculated that anti-DP antibodies seemed to be involved more frequently in poorer graft outcomes. Ling et al. investigated the clinical outcomes in kidney transplant recipients with isolated donor-specific anti-HLA-Cw antibodies2. They identified eight patients with pretransplant DSA anti-HLA-Cw, exclusively. During a 6-month median of follow-up (range 3–24 months), patient and graft survival was 100% without any acute rejection occurring. In this group, all the patients had induction therapy with thymoglobulin or basiliximab and additionally all patients received intravenous immunoglobulin, similar to patients with positive flow cytometry crossmatch (FCXM) and/or cPRA>50%. Even so, the median time of follow-up was relatively short and may have underestimated the incidence of rejection. Aubert et al. evaluated retrospectively 22 renal transplant recipients with isolated anti-HLA-Cw DSA at day 0 of renal transplant comparing them with 88 allo-sensitized patients with no preformed DSA (control group), and followed for a period of 1 year17. Acute AMR was diagnosed in six patients (27.3%) with DSA-Cw versus 9% in those without DSA. In this study, the patients with DSA anti-
HLA-Cw received less-intensive immunosuppression than the control group of immunized patients, including ATG induction (only 59.1%), and this may probably be a plausible explanation for this high rate of AMR. However, they alert for the necessity of screening pretransplant DSA HLA-Cw and subsequent modulation of immunosuppression in cases of positivity. More recently, Bachelet el al. analyzed the clinical impact of DSA anti-HLA-Cw and/or -DP through a retrospective study, comparing 48 patients transplanted with isolated preformed DSA anti-HLA-Cw and/or –DP with a group of HLA-sensitized recipients with no DSA (104 patients) and 47 kidney transplant recipients with preformed DSA anti-HLA-A, −B, −DR, and/or –DQ18. Two years after transplantation, the groups with DSA (both Cw/DP or A/B/DR/DQ) had similar incidence of AMR and graft survival (and worse than the group with no DSA), showing that preformed DSA anti–HLA-Cw and/or -DP were as deleterious as DSA anti–HLA A/B/DR/DQ.
Concerning immunological risk assessment in transplantation, we know that DSA levels measured as mean fluorescence intensity (MFI) and flow cytometry crossmatch channel shift values increased our ability to stratify the development of AMR; both techniques were performed in our patient. Another procedure that has prognostic value for AMR occurrence is the detection of complement-fixing anti-HLA antibodies, which is the first step in the activation of the classical complemente cascade, namely the Luminex C1q assay. Akalin et al. showed recently that C1q DSA was associated with acute and chronic AMR and is important in the identification of atients at risk for antibody-mediated allograft injury19. However in our patient this test was not performed since it is not part of the protocol and the costs are significant.
In our clinical case, despite induction immunosuppressive therapy with high dose of thymoglobulin, we witnessed an increase in the titer of preformed DSA anti-HLA Cw2 and the onset of de novo DSA soon after transplantation. We believe that the preformed DSA anti-HLA-Cw present before transplantation and the association with AMR occurrence is clear in our patient, with data from literature confirming that their presence is as deleterious as DSA anti–HLA A/B/DR/DQ. Therefore, HLA-C typing and respective antibody identification will benefit sensitized patients during organ allocation and will allow us to use induction therapy or desensitization strategies properly.
References
1. Bryan CF, Luger AM, Smith JL, Warady BA, Wakefield M, Schadde E, et al. Sharing kidneys across donor-service area boundaries with sensitized candidates can be influenced by HLA C. Clin Transplant. 2010;24(1):56-61. [ Links ]
2. Ling M, Marfo K, Masiakos P, Aljanabi A, Lindower J, Glicklich D, et al. Pretransplant anti-HLA-Cw and anti-HLA-DP antibodies in sensitized patients. Hum Immunol. 2012;73(9):879-83. [ Links ]
3. Thorsby E, Sandberg L, Lindholm A, Kissmeyer-Nielsen F. The HL-A system: evidence of a third sub-locus. Scand J Haematol. 1970;7(3):195-200. [ Links ]
4. Gilbert M, Paul S, Perrat G, Giannoli C, Pouteil Noble C, Morelon E, et al. Impact of pretransplant human leukocyte antigen-C and -DP antibodies on kidney graft outcome. Transplant Proc. 2011;43(9):3412-4. [ Links ]
5. Bachelet T, Couzi L, Guidicelli G, Moreau K, Morel D, Merville P, et al. Anti-Cw donorspecific alloantibodies can lead to positive flow cytometry crossmatch and irreversible acute antibody-mediated rejection. Am J Transpl2011;11(7):1543-4. [ Links ]
6. Duquesnoy RJ, Marrari M. Detection of antibodies against HLA-C epitopes in patients with rejected kidney transplants. Transpl Immunol. 2011;24(3):164-71. [ Links ]
7. Suneja M, Kuppachi S. Acute antibody-mediated renal allograft rejection associated with HLA-Cw17 antibody. Clin Kidney J. 2012;5(3):254-6. [ Links ]
8. Lefaucheur C, Loupy A, Hill GS, Andrade J, Nochy D, Antoine C, et al. Preexisting donorspecific HLA antibodies predict outcome in kidney transplantation. Journal of the American Society of Nephrology: JASN. 2010;21(8):1398-406. [ Links ]
9. Loupy A, Hill GS, Jordan SC. The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat Rev Nephrol. 2012;8(6):348-57. [ Links ]
10. Sis B, Mengel M, Haas M, Colvin RB, Halloran PF, Racusen LC, et al. Banff 09 meeting report: antibody mediated graft deterioration and implementation of Banff working groups. Am J Transpl. 2010;10(3):464-71. [ Links ]
11. Mohan S, Palanisamy A, Tsapepas D, Tanriover B, Crew RJ, Dube G, et al. Donor-specific antibodies adversely affect kidney allograft outcomes. Journal of the American Society of Nephrology: JASN. 2012;23(12):2061-71. [ Links ]
12. Tait BD, Susal C, Gebel HM, Nickerson PW, Zachary AA, Claas FH, et al. Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation. 2013;95(1):19-47. [ Links ]
13. Neisig A, Melief CJ, Neefjes J. Reduced cell surface expression of HLA-C molecules correlates with restricted peptide binding and stable TAP interaction. J Immunol. 1998;160(1):171-9. [ Links ]
14. McCutcheon JA, Gumperz J, Smith KD, Lutz CT, Parham P. Low HLA-C expression at cell surfaces correlates with increased turnover of heavy chain mRNA. The J Exp Med 1995;181(6):2085-95. [ Links ]
15. Chapman JR, Taylor C, Ting A, Morris PJ. Hyperacute rejection of a renal allograft in the presence of anti-HLA-Cw5 antibody. Transplantation. 1986;42(1):91-3. [ Links ]
16. Rogers NM, Bennett GD, Toby Coates P. Transplant glomerulopathy and rapid allograft loss in the presence of HLA-Cw7 antibodies. Transpl Int. 2012;25(3):e38-40. [ Links ]
17. Aubert O, Bories MC, Suberbielle C, Snanoudj R, Anglicheau D, Rabant M, et al. Risk of antibody-mediated rejection in kidney transplant recipients with anti-HLA-C donorspecific antibodies. Am J Transpl. 2014;14(6):1439-45. [ Links ]
18. Bachelet T, Martinez C, Del Bello A, Couzi L, Kejji S, Guidicelli G, et al. Deleterious Impact of Donor-Specific Anti-HLA Antibodies Toward HLA-Cw and HLA-DP in Kidney Transplantation. Transplantation. 2016;100(1):159-66. [ Links ]
19. Calp-Inal S, Ajaimy M, Melamed ML, Savchik C, Masiakos P, Colovai A, et al. The prevalence and clinical significance of C1q-binding donor-specific anti-HLA antibodies early and late after kidney transplantation. Kidney international. 2016;89(1):209-16. [ Links ]
Sofia Santos
Department of Nephrology
Centro Hospitalar do Porto – Hospital Geral de Santo António
Largo Prof. Abel Salazar 4099-001 PORTO – Portugal
E-mail: sofia.fersantos@gmail.com
Disclosure of potential conflicts of interest: none declared
Received for publication: Nov 11, 2016
Accepted in revised form: Jan 30, 2017














