Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Portuguese Journal of Nephrology & Hypertension
versión impresa ISSN 0872-0169
Port J Nephrol Hypert vol.31 no.1 Lisboa mar. 2017
ORIGINAL ARTICLE
Renal involvement in rheumatoid arthritis: analysis of 53 renal biopsies
Mário Góis, Fernanda Carvalho, Helena Sousa, Ana Carina Ferreira, João Sousa, Fernando Nolasco
Nephrology Department, Hospital Curry Cabral, Centro Hospitalar de Lisboa Central, Lisboa, Portugal
ABSTRACT
Background: Rheumatoid arthritis (RA) is a systemic inflammatory disorder characterized by joint inflammation, associated with autoantibody production. Renal involvement arises as a complication of treatment or can be related to the disease itself.
Methods: 53 biopsies from patients with RA from 1989 to 2015 were reviewed. Histologic diagnosis, age, gender, duration of RA, drug therapy, renal function, proteinuria and haematuria were analyzed.
Results: Amyloidosis was the most common renal histologic pattern (21 patients). Membranous Nephropathy (MN) was found in 12 patients, followed by Mesangial Proliferative Glomerulonephritis (n=4) and Focal and Segmental Glomerulosclerosis (n=4), IgA Nephropathy (n=3), Necrotizing Glomerulonephritis (n=3), Chronic Interstitial Nephritis (n=3), Endocapillary Proliferative Glomerulonephritis (n=2) and Minimal Change Disease (n=1). Amyloidosis correlated with long duration RA (14.9±6.66 years vs 8.84±6.37 years; p<0.001), presenting with nephrotic proteinuria in the majority of the cases (5.11±2.94 g/24h vs 3.52±2.71 g/24h p=0.03), which correlates with dominant glomerular amyloid deposition (7.0±2.28 g/24h vs 3.04±2.08 g/24h; p<0.001). In patients with MN, renal function was preserved (serum creatinine 0.83±0.21mg/dl vs 2.03±0.21mg/dl; p<0.001) and one third of the cases presented with haematoproteinuria. Disease modifying antirheumatic drugs (DMARDs) could be related with MN in six cases. Patients with Necrotizing Glomerulonephritis had a severe renal involvement, as did patients with Chronic Interstitial Nephritis.
Conclusion: We found a wide spectrum of histological lesions that cannot be predicted with only clinical and laboratory findings. Thus, renal biopsy is essential to ensure correct diagnosis in RA patients who present with urinary abnormalities or deteriorated renal function.
Key-words: Glomerulonephritis, renal biopsy, rheumatoid arthritis.
INTRODUCTION
Rheumatoid arthritis (RA) is an autoimmune disorder that affects primarily the joints. Extra-articular involvement may also occur, with appearance of rheumatoid nodules, pulmonary interstitial fibrosis, pulmonary nodules, pericarditis, mononeuritis multiplex, episcleritis and systemic vasculitis1. Renal findings in RA can be divided into three categories: secondary amyloidosis related to serum amyloid A protein (SAA); complications of treatment with disease modifying antirheumatic drugs (DMARDs), analgesics or non-steroidal anti-inflammatory agents (NSAIDs); renal disease related to RA itself or with an autoimmune predisposition2. The incidence of renal disease in RA is relatively low, but it causes significant morbidity and mortality, especially if renal amyloidosis is present3,4.
Renal histopathologic lesions are heterogeneous and cannot be predicted with clinical and laboratory findings only. Thus, renal biopsy plays a key role in ensuring correct diagnosis. In this study we analyzed renal involvement in 53 patients with RA.
PATIENTS AND METHODS
We studied renal biopsies of 57 patients, received by our laboratory from January 1989 to December 2015.
The diagnosis of RA was made in line with the 2010 RA classification criteria of the American College of Rheumatology/European League Against Rheumatism. We excluded four patients because their renal pathology was probably not related with RA or DMARDs – one patient with normal kidney, one patient with hypertensive nephroangiosclerosis, one patient with cast nephropathy and one patient with sclerotic lesions that could not be included in any histological diagnosis. Thus, we had a representative sample of 53 patients (34 female and 19 male). The mean age of the patients was 53.81 ± 15.91 years (range 16-87 years) and the mean RA duration was 11.2 ± 57.06 years (range 2-30 years).
Proteinuria was defined as protein urinary excretion of more than 300mg/24h and haematuria was defined as urine sedimentary red blood cell counts of more than five in the visual field of a x400 microscope.
Renal biopsy specimens were examined by light microscopy (LM) and immunofluorescence (IF). Electron microscopy was performed only in a patient who had a diagnosis of minimal change disease (MCD). The LM analysis was performed after staining with haematoxylin and eosin (HE), periodic acid Schiff (PAS), periodic acid methenamine silver (PAM), Massons trichrome and Congo-red. The IF evaluation was performed in frozen sections using fluorescein isothiocyanate conjugated antisera to human immunoglobulins (IgG, IgA, IgM), light chains (λ and κ), complement (C1q, C3), and fibrinogen. Amyloid substance was characterized using immunofluorescence or immunochemistry staining for SAA or κ and λ light chains.
We searched for a relationship between renal histologic findings and clinical data, such as gender, age, serum creatinine (Scr) and glomerular filtration rate estimated (eGFR) by Modification of Diet in Renal Disease (MDRD) study equation, haematuria, proteinuria, duration of RA and predominant location amyloid deposits (glomerular vs vascular).
Statistical analysis was performed using SPSS, version 22. We used mean and standard deviation to characterize quantitative variables, and qualitative variables were analyzed using absolute and relative frequencies.
The relationship between qualitative variables was accessed with the chi-square test. Quantitative variables were analyzed using the Mann-Whitney and Kruskal-Wallis tests. Non-parametric methods were used because of sample size and distribution type of quantitative variables. A p value of <0.05 was considered significant.
RESULTS
Renal histology
The most frequent diagnosis was renal amyloidosis (n=21). Twelve patients had membranous nephropathy (MN) and the remaining patients had heterogeneous renal findings, with similar frequency (Table I).
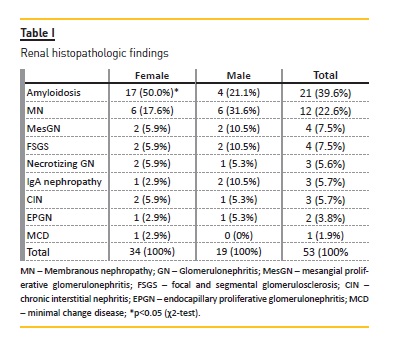
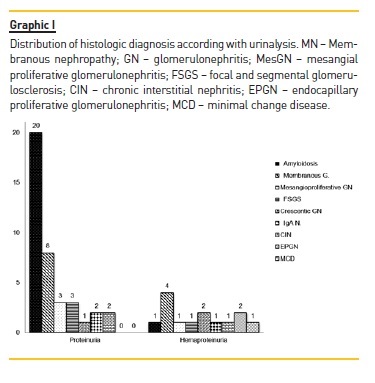
All patients had proteinuria, however only 14 (26.4%) had microhaematuria (haematoproteinuria group). The relation between urinalysis and renal histopathologic findings are shown in Graphic I.
Mean Scr, eGFR and duration of RA varied according to the underlying histologic pattern, while age and degree of proteinuria were similar between groups. (Table II).
Proteinuria was equal or more than 3.5g/24h in 49% patients (n=26) and, in this group, 19 patients had also nephrotic syndrome (Table III).
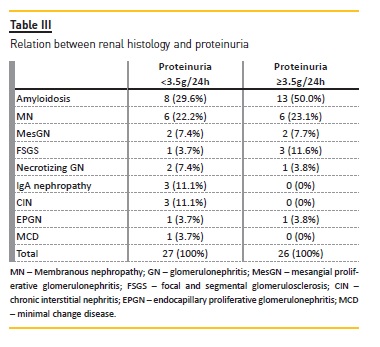
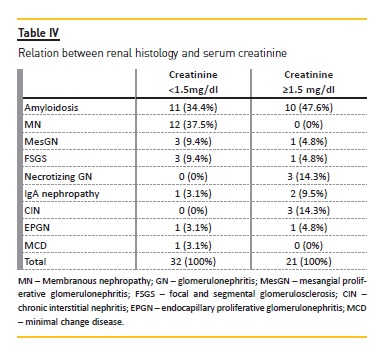
Table IV represents the relationship between renal function and renal histology. Twenty-one patients had Scr ≥1.5mg/dl, and the majority of them had renal amyloidosis (n=10). All patients with MN had Scr <1.5mg/dl.
Clinical and pathologic features of each histologic pattern
1. Amyloidosis
Amyloidosis was the most frequent renal finding (39.6%). Only amyloid A deposits were detected (AA amyloidosis). The majority of these patients were female (n=17; p=0.03), and presented mostly with nephrotic proteinuria (5.11±2.94 g/24h vs 3.52±2.71 g/24h; p=0.03), including 12 with nephrotic syndrome.
Only one patient presented with haematuria. This diagnosis mainly occurred in patients with long duration RA (14.9±6.66 years vs 8.84±6.37 years; p<0.001) and in older patients (56.48±17.69 years), but renal function was only slightly deteriorated: Scr 1.67±1.16mg/dl and eGFR 57.29±39.58 ml/min/1.73m2. Amyloid deposits were located in glomeruli, arteries and interstitium (two cases). The patients with dominant glomerular amyloid deposition (n=11) had significantly higher levels of proteinuria (7.0±2.28 g/24h vs 3.04±2.08 g/24h; p<0.001) and more severe deterioration of renal function (2.03±1.41mg/dl vs 1.28±0.68mg/dl; p=0.173), than those with predominantly vascular deposits.
2. Membranous Nephropathy
MN was diagnosed in 12 patients. All patients had proteinuria (six in nephrotic range, but only four patients had nephrotic syndrome) and four also had microhaematuria. Renal function was mostly preserved and all patients were biopsied with Scr <1.5mg/dl (0.83±0.21mg/dl vs 2.03±0.21mg/dl; p<0.001). MN occurs in younger patients (47±14.96 years) with short duration RA (6.91±3.03 years). MN appeared to be related with penicillamine in two cases and with gold sodium thiomalate in four cases. According to Ehrenreich and Churgs classification, two patients had MN in stage I, and ten patients in stage II. All six patients with MN associated with DMARDs were lost to followup.
Circulating anti-phospholipase A2 receptor autoantibodies were found in the remaining six patients. With regard to the treatment, all these patients achieved complete remission (proteinuria <300mg/24h): two patients with a 1-year treatment with cyclosporine; four patients with 6-month treatment with cyclophosphamide. No relapses were detected.
DISCUSSION
The renal histologic findings found in this study were similar to that reported in previous studies5-9. AA Amyloidosis was the most common diagnosis, followed by MN. Reported as the most frequent diagnosis in several studies, we found MesGN only in 7.5% of cases, presenting with proteinuria in three cases and haematoproteinuria in one case. Both patients with nephrotic proteinuria were treated with gold sodium thiomalate.
Korpela et al. reported 23 patients with MesGN that presented with haematuria (ten patients), isolated proteinuria (six patients) and haematoproteinuria (seven patients) with normal renal function in most of the cases. They determined that mesangial lesion presenting with proteinuria was associated to DMARD (mainly gold and penicillamine) and was reversible on drug withdrawal. However, the mesangial proliferation presenting with isolated haematuria was not linked to any therapy and was persistent, even with discontinuation of DMARD10. By IF evaluation, our cases of MesGN had mainly IgM with few mesangial deposits of C3 and it was similar to other previous reports. In 40 cases of MesGN reported by Helin et al8,10, mesangial deposits were mainly IgM (12 specimens), associated with lesser amounts of IgA, IgG and C3. In RA, the high levels of IL-611 promote proliferation of mesangial cells and it has been proposed as a pathogenic pathway to explain mesangial proliferation in kidneys of RA patients12. Pollet et al. found association between IgM rheumatoid factor (IgM-RF) and MesGN, and suggested that mesangial lesions are a consequence of a functional response by renal mesangium to remove immunocomplexes formed by IgM-RF and IgG13. We detected elevated titers of serum rheumatoid factor, but its immunoglobulin class was not evaluated.
We described three patients with IgA Nephropathy who had elevated serum IgA levels and elevated rheumatoid factor (not characterized). IgA nephropathy has been associated with RA due to: (1) common pathogenic pathways, including HLA-DR414; (2) increased IgA levels in both diseases15; (3) IgA-rheumatoid factor16.
AA amyloidosis was the most frequent diagnosis in our case series. These patients had higher levels of proteinuria and, rarely, haematuria, and occurred mainly with long-duration RA. As expected, in cases of glomerular dominant amyloidosis, proteinuria was higher than in patients with dominant vascular deposits.
Compared to previous studies, we report a higher frequency of amyloidosis. This is explained by many reasons: (1) the main indication for renal biopsy was proteinuria; (3) patients were older; (2) patients had a long duration RA. However, analyzing the last 10 years, we found only 1 patient with AA amyloidosis.
The incidence of amyloidosis in RA patients seems to be decreasing, possibly as consequence of more effective inflammation control with new therapeutic agents17. There is no specific treatment for AA amyloidosis, but control of inflammation can lead to better outcomes. If the level of SAA is maintained at less than 10mg/L, the 10-year survival rate increases to 90%, but when SAAL levels are more than 10mg/L, this rate decreases to less than 40%18. Recently, anti-TNF-α agents etanercept19, infliximab20, and tocilizumab (a humanized anti-IL-6 receptor antibody)21 have shown efficacy in the treatment of RA-associated AA amyloidosis.
A promising drug is eprodisate, a sulfonated molecule with structure similar to heparin sulfate, which competitively binds to glycosaminoglycan-binding sites on SAA and inhibits fibril polymerization and amyloid deposition. A recent randomized clinical trial showed that eprodisare may reduce progression in AA amyloidosis22.
The second most frequent diagnosis was MN (22.6%).
In half of patients, MN seems to be associated with DMARD (4 with gold sodium thiomalate and 2 with penicillamine). In our experience, the patients with MN had normal renal function in accordance with other studies. Makino et al. described 100 patients with RA and renal disease. Thirty-one patients had MN and presented with preserved renal function. Twenty-four cases of MN were presumptively related with DMARD23.
Gold therapy (oral or parental administration) is complicated with proteinuria in 10% of patients and about 85% of patients develop proteinuria (range 0.7g – 30g) within the first 24 months of treatment, including nephrotic syndrome in one third of cases (in our study 4 of 12 patients had nephrotic syndrome)24,25. In most of the cases, the proteinuria remits when DMARDs are stopped, but in some cases the proteinuria persists26.
Proteinuria can be detected in about 30% of patients treated with penicillamine and resolves after drug withdrawal27.
MN associated with penicillamine and gold has the same histologic pattern as idiopathic MN. The pathogenesis in both cases is unknown, but it was proposed an association with HLA-DRw3 and HLA-B828.
Gold is a tubular toxin and, in one study, it was suggested that damaged tubules could release antigens that induce an autoantibody response to related podocyte proteins29.
Necrotizing GN was found in three patients and all had positive MPO-ANCA. It has been described the association between necrotizing GN and RA, and the majority of these patients had positive MPO-ANCA and extra-renal manifestations30. This type of renal limited vasculitis has also been described in association with penicillamine31 and anti-TNF- α agents32. In our experience, all patients responded well to the treatment (two cases treated with cyclophosphamide and one case successfully treated with rituximab).
Chronic Interstitial Nephritis is historically correlated with NSAIDs and analgesic consumption. Our three cases presented with low-grade proteinuria, reflecting slight glomerular damage, probably secondary to severe tubular lesions. Lack of alterations in urinalysis delay the diagnosis, which could explain why this group of patients had higher Scr. Although only three patients were diagnosed with Chronic Interstitial Nephritis, tubule-interstitial lesions were detected in 35 patients, characterized by tubular atrophy (52%), mononuclear cell infiltration (32%) and interstitial fibrosis (78%), explained not only by nephrotoxicity of NSAIDs and analgesic drugs, but also by cyclosporine and, at least, by massive proteinuria secondary to glomerular lesions.
Our work has several limitations. This is a retrospective study and the analysis is based on biopsies performed on the basis of clinical criteria and does not represent the entire renal pathology in RA patients.
Another important limitation concerns the absence of analysis by electron microscopy.
This study shows that kidney disease in RA patients has a wide spectrum. Renal biopsy is essential to diagnosis and prognosis for the reason that isnt possible predict with good accuracy histologic findings only with clinical and laboratory data. Over the last decade, the understanding of RA pathogenesis has led to the development of new biologic agents, which have dramatically changed the treatment, with improvements in RA symptoms, slowed disease progression and improved physical function and quality of life for patients33. Its expected that the effective control of disease can lead to the avoidance of renal complications of chronic systemic inflammation, such as secondary amyloidosis, and change the incidence of renal disease in RA.
References
1. Turesson C, Jacobsson L, Bergstrom U. Extra-articular rheumatoid arthritis: prevalence and mortality. Rheumatology (Oxford) 1999;38(7):668-674. [ Links ]
2. Boers M, Croonen AM, Dijkmans BA, et al. Renal findings in rheumatoid arthritis: clinical aspects of 132 necropsies. Ann Rheum Dis 1987;46(9):658-663. [ Links ]
3. Sihvonen S, Korpela M, Mustonen J, et al. Renal disease as a predictor of increased mortality among patients with rheumatoid arthritis. Nephron Clin Pract 2004;96(4):c107-c114. [ Links ]
4. Mitchell DM, Spitz PW, Young DY, et al. Survival, prognosis, and causes of death in rheumatoid arthritis. Arthritis Rheum 1986;29:706-714. [ Links ]
5. Salomon MI, Gallo G, Poon TP, Goldblat MV, Tchertkoff V. The kidney in rheumatoid arthritis: a study based on renal biopsies. Nephron 1974;12:297-310. [ Links ]
6. Ojavik 0, Brodvall EK, Oystese B, Natvik JB, Mellbye OJ. A renal biopsy study with light and immunofluorescence microscopy in rheumatoid arthritis. Acta Med Scand Suppl 1981;6459-14. [ Links ]
7. Helin H, Korpela M, Mustonen J, Pasternack A. Mild mesangial glomerulopathy: a frequent finding in rheumatoid arthritis patients with hematuria or proteinuria. Nephron 1986;42:224-230. [ Links ]
8. Boers M, Croonen AM, Dijkmans BA, et al. Renal findings in rheumatoid arthritis: clinical aspects of 132 necropsies. Ann Rheum Dis 1987;46(9):658-663. [ Links ]
9. Helin HJ, Korpela MM, Mustonen JT, et al. Renal biopsy findings and clinicopathologic correlations in rheumatoid arthritis. Arthritis Rheum 1995;38(2):242-247. [ Links ]
10. Korpela M, Mustonen J, Pasternack A, et al. Mesangial glomerulopathy in rheumatoid arthritis patients. Clinical follow-up and relation to antirheumatic therapy. Nephron 1991;59(1):46-50. [ Links ]
11. Kishimoto T. The biology of interleukin-6. Blood 1989;74:1-10. [ Links ]
12. Horii Y, Muraguchi A, Iwano M, et al. Involvement of IL-6 mesangial proliferative glomerulonephritis. J Immunol 1989;143:3949-3955. [ Links ]
13. Pollet S, Depner T, Moore P, Olander H, Robbins D. Mesangial glomerulopathy and IgM rheumatoid factor in rheumatoid arthritis. Nephron 1989;51:107-111. [ Links ]
14. Feehally J, Farral M, Boland A, et al. HLA has strongest association with IgA nephropathy in genome-wide analysis. J Am Soc Nephrol 2010;21:1791-1797. [ Links ]
15. LJ Badcock, Clarke S, PW Jones, Dawes PT, Mattey DL. Abnormal IgA levels in patients with rheumatoid arthirits. Arthritis Rheum Dis 2003;62:83-84. [ Links ]
16. Bas S, Genevay S, Meyer O, Gabay C. Anti-cyclic citrullinated peptide antibodies, IgM and IgA rheumatoid factors in the diagnosis and prognosis of rheumatoid arthritis. Rheumatology 2003;42(5):677-680. [ Links ]
17. Immonen K, Finne P, Gronhagen-Riska C, et al. A marked decline in the incidence of renal replacement therapy for amyloidosis associated with inflammatory rheumatic diseases – data from nationwide registries in Finland. Amyloid 2011;18(1):25-28. [ Links ]
18. Gillmore JD, Lovat LB, Persey MR, et al. Amyloid load and clinical outcome in AA amyloidosis in relation to circulating concentration of serum amyloid A protein. Lancet 2001;358:24-29. [ Links ]
19. Nakamura T, Higashi S, Tomoda K, et al. Etanercept can induce resolution of renal deterioration in patients with amyloid A amyloidosis secondary to rheumatoid arthritis. Clin Rheumatol 2010;29(12):1395-1401. [ Links ]
20. Keersmaekers T, Claes K, Kuypers DR, et al. Long-term efficacy of infliximab treatment for AA-amyloidsis secondary to chronic inflammatory arthritis. Ann Rheum Dis 2009;68(5):759-761. [ Links ]
21. Nishida S, Hagihara K, Shima Y, et al. Rapid improvement of AA amyloidosis with humanized anti-interleukin 6 receptor antibody treatment. Ann Rheum Dis 2009;68(7):1235-1236. [ Links ]
22. Manenti L, Tansinda P, Vaglio A. Eprodisate in AA amyloidosis. N Engl J Med 2007; 357(11):1153. [ Links ]
23. Makino H, Yashinaga Y, Yamasaki Y, Morita Y, Hashimoto H, Yamamura M. Renal involvement in rheumatoid arthritis: analysis of renal biopsy specimens from 100 patients. Mod Rheumatol 2002;12:148-154. [ Links ]
24. Lockie LM, Smith DM. Forty-seven years experience with gold therapy in 1,019 rheumatoid arthritis patients. Semin Arthritis Rheum 1985;14(4):238-246. [ Links ]
25. Hall CL, Fothergill NJ, Blackwell MM, et al. The natural course of gold nephropathy: long term study of 21 patients. Br Med J (Clin Res Ed) 1987;295(6601):745-748. [ Links ]
26. Silverberg DS, Kidd EG, Shnitka TK, et al. Gold nephropathy. A clinical and pathologic study. Arthritis Rheum 1970;13(6):812-825. [ Links ]
27. Hall CL, Jawad S, Harrison PR, et al. Natural course of penicillamine nephropathy: a long term study of 33 patients. Br Med J (Clin Res Ed) 1988;296(6629):1083-1086. [ Links ]
28. Wooley PH, Griffin J, Panayi GS, et al. HLA-DR antigens and toxic reaction to sodium aurothiomalate and D-penicillamine in patients with rheumatoid arthritis. N Engl J Med 1980;303(6):300-302. [ Links ]
29. Ueda S, Wakashin M, Wakashin Y, et al. Experimental gold nephropathy in guinea pigs: detection of autoantibodies to renal tubular antigens. Kidney Int 1986;29(2):539-548. [ Links ]
30. Harper L, Cockwell P, Howie AJ, et al. Focal segmental necrotizing glomerulonephritis in rheumatoid arthritis. Q J Med 1997;90:125-132. [ Links ]
31. Bienaimé F, Clerbaux G, Plaisier E, Mougenot B, Ronco P, Rougier JP. D-Penicillamineinduced ANCA-associated crescentic glomerulonephritis in Wilson disease. Am J Kidney Dis 2007;50(5):821-825. [ Links ]
32. Doulton TW, Tucker B, Reardon J, et al. Antineutrophil cytoplasmic antibody-associated necrotizing crescentic glomerulonephritis in a patient receiving treatment with etanercept for severe rheumatoid arthritis. Clin Nephrol 2004;62(3):234-238. [ Links ]
33. Curtis JR, Singh JA. The use of biologic in rheumatoid arthritis: current and emerging paradigms of care. Clin Ther 2011;33(6):679-707. [ Links ]
Mário Góis
Nephrology Department
Hospital Curry Cabral, Centro Hospitalar de Lisboa Central
Rua da Beneficência n. 8 1600-166 Lisboa, Portugal.
E-mail: mariovgois@gmail.com
Disclosures of potential conflicts of interest: None declared.
Received for publication: Nov 14, 2016
Accepted in revised form: Mar 8, 2017