Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.30 no.4 Lisboa dez. 2016
CASE REPORT
A rare malignancy in the post-transplant period: Myeloma cast nephropathy
Isabel Mesquita1, Helena Viana1, Marília Possante1, Cristina João2, Susana Carvalho2, Cecília Silva1, Inês Aires1, Aníbal Ferreira1, Francisco Remédio1, Fernanda Carvalho1, Fernando Nolasco1
1 Hospital Curry Cabral do CHLC, Serviço de Nefrologia e Transplantação, Lisboa, PT
2 Instituto Português de Oncologia, Serviço de Hematologia, Lisboa, PT
ABSTRACT
Myeloma-associated renal disorders are rare events among renal transplants and can occur as recurrent or de novo disease. We describe an unusual case of renal allograft dysfunction due to myeloma cast nephropathy occurring 19 years after having received a renal transplant in a patient with no prior history of multiple myeloma or monoclonal gammopathy preceding transplantation. Our patient was treated with five cycles of chemotheraphy (bortezomib, melphalan and steroids), which resulted in short-term improvement in allograft dysfunction and complete haematological remission. The longer patient and graft survival after renal transplantation make post-transplant lymphoproliferative disease more frequent. Multiple myeloma after kidney transplant is rare and an elevated index of suspicion is necessary to make a timely diagnosis of this serious disease. Further work is needed to identify the best treatment options for these patients.
Key-Words: Kidney transplantation; myeloma cast nephropathy; post-transplant lymphoproliferative disease.
INTRODUCTION
Kidney transplantation has experienced dramatic developments over the years, in the treatment and is now the standard treatment for patients with end-stage renal disease1.
An increased incidence of neoplasm in transplant recipients was first recognized in the 1970s as a serious complication2. With longer graft survival and older donors (as well as recipients) and with the introduction of more potent immunosuppressive medication, cancers complicating organ allograft are a major cause of morbidity and mortality after renal transplantation2,3.
In published studies, the incidence of cancer in renal transplant recipients ranges from 2.3-31%3,4.
Of these, post-transplant lymphoproliferative disease (PTLD) is a serious complication of solid organ transplantation, with an increased risk compared to both the general population and patients on transplant waiting lists5,6.
Factors predisposing to this increased risk of malignancy include impaired immune surveillance due to use of high-dose immunosuppressive drugs; primary infection or recrudescence of viral infections, such as Epstein-Barr virus, Herpes virus and hepatitis C virus (HCV), and older age5,7-9.
In the United States, approximately around 1% of all cancers and 10% of haematological malignancies in the general population are due to multiple myeloma10,11.
However, despite the frequency of multiple myeloma, there is limited experience with multiple myeloma in renal transplantation11.
Myeloma-associated kidney disorders occur as recurrent disease, and rarely de novo in kidney transplant recipients11,12. These kidney disorders usually manifest with worsening allograft function and proteinuria13.
Little is known about the course and outcome of patients who develop multiple myeloma after kidney transplantation. In the first published cases7,13-15 the outcome was poor because the therapeutic regimen with alkylator-based therapy used in an immunosuppressed population often resulted in severe infection and death. We believe that this scenario is now quite different with the advent of new myeloma therapies and with the use of less aggressive immunosuppressive protocols.
We report the case of one patient who developed multiple myeloma 19 years after having received a renal transplant.
CASE REPORT
A 51-year-old Caucasian female with an autosomal dominant polycystic kidney disease was transplanted with a deceased allograft kidney in 1993. Her past medical history was significant for hypertension and HCV.
There was no clinical evidence of multiple myeloma at that time. There were four HLA mismatches (A, B and DR). The surgical procedure was uneventful and immunosuppression (ISS) consisted of prednisolone, cyclosporine and azathioprine. The optimal plasma creatinine after transplantation was 1.0 mg/dl and the patient was diagnosed at discharge with new onset diabetes after transplantation.
In the first two years after transplantation patient experienced cytomegalovirus infection, treated with ganciclovir, and several Escherichia coli urinary tract infections (UTI). In the third year post-transplantation, renal function declined to a plasma creatinine 1.5 mg/dl. An allograft biopsy was made at this point and showed interstitial fibrosis and tubular atrophy and arteriolar hyaline thickening, suggesting calcineurin inhibitor toxicity. The cyclosporine dose was reduced.
In the fifth year after transplantation, azathioprine was switched to mycophenolate mofetil.
At 7th year post-transplantation the patient was diagnosed with vesicoureteral reflux into the transplanted kidney during investigation of underlying causes of recurrent UTI. She was treated by endoscopic subureteral collagen injection.
Nine years after transplantation she started erythropoiesis-stimulating agents (ESA) to achieve normochromic normocytic anaemia control (darbepoetin alfa 20 mcg, biweekly). In the thirteenth year after transplantation, cyclosporine was switched to tacrolimus as cyclosporine-related minor side effects were observed.
Over the next few years, follow-up visits in the transplant clinic were unremarkable except for several UTI and an increase in ESA dose (from 20 to 80 mcg, weekly) to correct anaemia. The allograft function remained stable (Scr 1.3 mg/dl).
At the follow-up visit during the 19th year posttransplant the patient presented with asthenia and fatigue at minimal efforts. At the time her ISS consisted of tacrolimus (1.5 mg/day) and mycophenolate mofetil (750 mg/day). Mild graft dysfunction was present with a slight creatinine increase (from 1.4 to 1.9 mg/dl).
Laboratory work-up revealed haemoglobin of 11 g/dl, total leukocyte count of 5800/mm3 and a normal differential count. Serum albumin was 3.6 g/dl, serum calcium 9.2 mg/dl, phosphorus 5.8 mg/dl and alkaline phosphatase 73 U/L. Serum protein electrophoresis revealed the presence of de novo M band and urinary Bence Jones proteins were positive. Serum immunoelectrophoresis showed immunoglobulin A (IgA) lambda monoclonal gammopathy of 1819 mg/dL and presence of urine protein electrophoresis was positive for an abnormal lambda monoclonal band.
An allograft kidney biopsy was performed due to mild creatinine increase and IgA lambda gammopathy.
The optical microscopy revealed normal glomeruli and vessels. The majority of proximal tubules were normal.
The more profound cortical and the medullar region presented interstitial fibrosis, with mild mononuclear cells infiltrates and tubular atrophy with polymorphic, dense and fragmented tubular casts. One tubular cast was involved by a multinucleated giant cell (Figure 1).
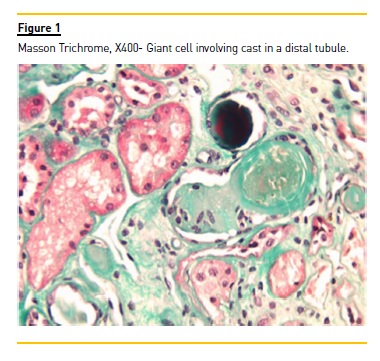
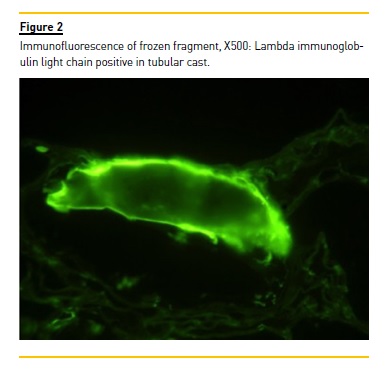
The immunofluorescence, performed in frozen fragment, was only positive to immunoglobulin lambda light chain in tubular casts (Figure 2). The Congo Red coloration was negative. Lambda cast nephropathy was the final histological diagnosis.
Subsequent bone marrow showed 30% mature and immature plasma cells, confirming the diagnosis of myeloma. Skeletal survey revealed one osteolytic lesion in the skull. The patient was referred to haematology and started 5 weekly cycles of chemotherapy.
Each cycle consisted of 4 days of bortezomib (1.3mg/m2 daily), 4 days of melphalan (9 ml/m2 daily) and 4 days of oral dexamethasone (40 mg daily). She received a total of 5 cycles of chemotherapy, resulting in a complete haematological remission represented by normal IgA lambda levels, negative immunofixation and recovery of baseline graft function. During chemotherapy, she developed leukopaenia and mycophenolate mofetil was withdrawn, maintaining tacrolimus monotherapy.
The patient continued to be followed-up at the haematology and transplant clinic, with monitoring of immunoelectrophoresis and free light chain.
Five months after the last treatment, tacrolimus was switched to everolimus. This caused proteinuria worsening which led to everolimus suspension and tacrolimus re-introduction.
Eighteen months after last chemotherapy cycle she remains in complete haematological remission with a stable kidney function and serum creatinine level sustained at 1.3 mg/dL.
Her current immunosuppression consists of tacrolimus and mycophenolate mofetil.
Presently, she is 74 years old, 21 years after kidney transplantation and remains in complete haematological remission with stable allograft function.
DISCUSSION
PTLD are well-known complication after renal transplantation and occur more frequently in situations where an aggressive immunosuppressive protocol has been used. In PTLP spectrum, patients with malignant lymphomas/multiple myeloma are older, develop the lesions after a longer time interval after transplantation18, have a higher stage of disease at presentation and lower probability of response to implemented therapeutic strategies8.
Myeloma-associated kidney disorders have been reported with diverse clinical and histological manifestations in solid organ transplant recipients15,19. This entity usually occurs as recurrent disease, and rarely de novo in kidney transplant recipients. The risk of developing de novo myeloma after renal transplantation is 4.4 times higher than in the general population; however, this accounts for only 1.3% of all malignancies over a period exceeding 25 years, per the ANZDATA report8,20.
It is possible that in some cases a previous undetected monoclonal gammopathy of undetermined significance (MGUS), preexisting prior to transplantation, could evolve into manifest myeloma post-transplant as a result of the immunosuppressive regimen.
In cases of known and treated myeloma, the disease could recur despite achieving successful remission prior to transplantation15.
Presence of monoclonal or oligoclonal gammopathies is, however, not infrequent after transplantation.
Incidence of benign gammopathies after transplantation varies between 10-30%7. Post-transplant MGUS is often transient, usually associated with intense immunosuppression, and the amount of M protein is usually small, and it may have multiple bands11,21.
Light chains (LCs) are removed from the circulation by the kidney. Filtered LCs are reabsorbed by proximal tubule epithelial cells via receptor-mediated endocytosis via the tandem endocytic receptors megalin/cubilin22. Excessive endocytosis of LCs triggers activation of nuclear factor jB, which in turn promotes cytokine and chemokine expression leading to tubulointerstitial fibrosis and scarring24.
The potential precipitants for myeloma cast nephropathy in post-transplant patients include calcineurin inhibitor toxicity, prolonged cold ischaemia time, transient hypovolaemia, and sudden exposure of the allograft to a high load of filtered free monoclonal light chains23.
Although kappa LCs are more commonly reported, there are few cases11,15 reporting lambda LC involvement in a renal allograft. It is not known if these pathological differences have significance in clinical outcome and allograft survival.
Renal insufficiency is common in multiple myeloma and is present in almost 50% of patients at the time of diagnosis. There is a negative correlation between the presence and severity of renal disease and patient survival25.
The response of the renal disease to therapy also appears to have prognostic value24,26. The proteasomal inhibitor bortezomib has represented a significant therapeutic step forward in the treatment of multiple myeloma since its approval in 2003. Bortezomib is associated with significant improvements in response rates, complete remission rates, event-free survival, patient survival and renal recovery rates24,27. Other chemotherapeutic agents with increasing use in patients include thalidomide and lenalidomide, presenting similar results to bortezomib26.
The role for therapeutic plasma exchange (TPE) in treatment of myeloma cast nephropathy remains controversial with contrasting results from different studies28-30, although the largest randomized controlled trial of TPE did not demonstrate any benefit30. Extended daily high cut-off haemodialysis (HCO-HD) has also been shown to allow removal of large amounts of serum free light chains31. Nevertheless, the studies only report reduction in free LC concentrations with HCO-HD in combination with chemotherapy. Currently is not known whether HCO-HD offers any additional benefit over current chemotherapeutic regimens. Randomized trials are in progress in this regard31.
There are no definite treatment protocols for patients with multiple myeloma who are eligible for kidney transplantation. Kuypers et al.32 have reported a case in which rituximab, an anti CD20 monoclonal antibody, delayed the recurrence of LC nephropathy in the allograft kidney. Some authors believe that dose-intensive chemotherapy / high dose intensity of chemotherapy followed by stem cell transplantation could decrease disease recurrence in the allograft kidney33.
The reported cases of myeloma-associated renal disorders in the transplant population are rare, with less than 50 reported. The described cases presentations were primary allograft dysfunction13,14,16,17,24 or renal impairment several months or years after renal transplantation7,11,15. In both presentations forms, allograft survival is poor. In 2011, Goel et al.24 published the first recovery of graft function. The patient had an established diagnosis of primary allograft dysfunction due to allograft myeloma cast nephropathy and had been treated with plasmapheresis, chemotheraphy using bortezomib and dexamethasone. Before that, Solak et al.34 reported a haematological remission without graft recovery in a patient with post-transplant myeloma treated with bortezomib.
The largest series, of seven patients with multiple myeloma after undergoing kidney transplantation, was recently published11. The authors showed that there is significant renal involvement in these patients and patient and renal allograft survival were not worse than those of the general population with multiple myeloma and kidney transplantation, respectively. However, the authors call attention to the fact that these results should be interpreted with caution because of the small sample size and that those patients who receive kidney transplantation are highly selected, which probably introduces bias11.
Our case illustrates the rare occurrence of multiple myeloma IgA lambda in a renal transplant recipient.
The presence of lambda LC in casts is uncommon since most published cases showed involvement of kappa LC. This is the first case of post-transplant de novo multiple myeloma seen in 1180 renal transplant patients followed up at our center.
The patient has responded to the implemented treatment of triple chemotherapy with dexamethasone, melphalan and bortezomib. Despite her advanced age and prolonged graft survival, treatment response was excellent. She achieved sustained complete haematological remission and returned to her basal allograft function. There were no complications associated with chemotherapy. This case is a strong argument that bortezomib could contribute to changing these patients outcomes.
An elevated index of suspicion is necessary to make a timely diagnosis of this serious disease, which probably determines a better prognosis24. Anaemia and increased need for ESA were the early signs of disease.
This case also raises the question of the routine use of protein serum electrophoresis as part of the posttransplant evaluation. Working together with Haematology was also critical to the success achieved.
In conclusion, although there are only a few cases described in the literature of this entity, we should not forget its existence, so we promote a rapid therapeutic intervention. In the last years, advances in treatment have led to an improvement of renal and vital prognosis.
References
1. Lamb KE, Lodhi S, Meier-Kriesche HU. Long-term renal allograft survival in the United States: a critical reappraisal. Am J Transplant 2011;11: 450–62. [ Links ]
2. Morath C et al. Malignancy in Renal Transplantation. J Am Soc Nephrol 2004;15: 1582–1588 [ Links ]
3. Dantal J, Pohanka E. Malignancies in renal transplantation: an unmet medical need. Nephrol Dial Transplant 2007; 22 (Suppl 1): i4–i10 [ Links ]
4. Andres A. Cancer incidence after immunosuppressive treatment following kidney transplantation. Crit Rev Oncol Hematol 2005; 56: 71–85 [ Links ]
5. Pascual J. Post-transplant lymphoproliferative disorder–the potential of proliferation signal inhibitors. Nephrol Dial Transplant 2007; 22 (Suppl 1):i27–i35 [ Links ]
6. Pascual J, Torrelo A, Teruel JL et al. Cutaneous T cell lymphomas after renal transplantation. Transplantation 1992; 53:1143–1145 [ Links ]
7. Gundappa RK, Sud K, Kohli HS, Jha V, Gupta KL, Sakhuja V. Multiple Myeloma: A rare malignancy in the post transplant period. Indian J Nephrol 2001; 11:66-67 [ Links ]
8. London NJ, Farmery SM, Will EJ, Davison AM, Lodge JP. Risk of neoplasia in renal transplant patients. Lancet 1995; 346:403-406 [ Links ]
9. Végsõ G, Hadju M, Sebestyén A. Lymphoproliferative Disorders After Solid Organ Transplantation–Classification, Incidence, Risk Factors, Early Detection and Treatment Options. Pathology & Oncology Research 2011; 17: 443-454 [ Links ]
10. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014; 64:9 [ Links ]
11. Safadi S, Dispenzieri A, Amer H et al. Multiple myeloma after kidney transplantation. Clin Transplant 2015; 29:76–84 [ Links ]
12. Caillard S. Myeloma, Hodgkin disease, and lymphoid leukemia after renal transplantation: characteristics, risk factors and prognosis. Transplantation 2006; 81: 888 [ Links ]
13. Perkowska-Ptasinska A, Glyda M, Paczkowski M, Durlik M. Primary Kidney Allograft Dysfunction Due to Myeloma-Cast Nephropathy: A Case Report Transplantation Proceedings 2007; 39,1683–1684 [ Links ]
14. Sammett D, Dagher F, Abbi R, Tomasula JR, Delaney V, Butt KM. Renal transplantation in multiple myeloma: case report and review of the literature. Transplantation 1996; 62:1577 [ Links ]
15. Balamuthusamy S, Hamrahian M, Zhang R, Batuman V. Myeloma kidney with isolated tubulointerstitial light chain deposition in a renal allograft. Clin Transplant 2009; 23:848–852 [ Links ]
16. Geiger X, Harris D, Van Buren D, et al: A middle-aged woman with refractory graft dysfunction in the early posttransplant period. Am J Kidney Dis 33:998, 1999 [ Links ]
17. Foster K, Cohen DJ, DAgati VD, et al: Primary renal allograft dysfunction. Am J Kidney Dis 2004; 44:376 [ Links ]
18. Nalesnik MA, Plasma cell tumors in transplant patients. Blood, 2013; 121, 8 [ Links ]
19. Lin J, Markowitz GS, Valeri AM et al. Renal monoclonal immunoglobulin deposition disease. The disease spectrum. J Am Soc Nephrol 2001; 12:1482 [ Links ]
20. ANZDATA Report: Australia and New Zealand Dialysis and Transplant Registry, Disney APS (ed): Adelaide, South Australia, 1991, p 95 ANZDATA A, Zealand N2006 Dialysis and Transplant Registry. ANZDATA Registry, Woodville [ Links ]
21. Radl J, Valentijn RM, Haaijman JJ, Paul LC. Monoclonal gammopathies in patients undergoing immunosuppressive treatment after renal transplantation. Clin Immunol Immunopathol 1985;37:98 [ Links ]
22. Batuman V, Guan S. Receptor-mediated endocytosis of immunoglobulin light chains by renal proximal tubule cells. Am J Physiol 1997; 272(4 Pt 2): F521 [ Links ]
23. Sengul S, Simon EE, Batuman V. Endocytosis of light chains induces cytokines through activation of NF-kappaB in human proximal tubule cells. Kidney Int 2002;62: 1977 [ Links ]
24. Goel S, Granger D, Bellovich K, Marin M, Qu H, El-Ghoroury M. Myeloma Cast Nephropathy: A Rare Cause of Primary Renal Allograft Dysfunction. Transplantation proceedings. Elsevier, 2011; 43:2784 [ Links ]
25. Korbet SM, Schwartz MM. Multiple Myeloma. J Am Soc Nephrol 2006;17:2533–2545 [ Links ]
26. Heher EC, Rennke HG, Laubach JP, et al. Kidney disease and multiple myeloma. Clin J Am Soc Nephrol 2013; 8:2007-2017 [ Links ]
27. Mateos MV, Hernandez JM, Hernandez MT, et al: Bortezomib plus melphalan and prednisone in elderly untreated patients with multiple myeloma: updated time-to-events results and prognostic factors for time to progression. Haematologica 2008;93:560 [ Links ]
28. Zuchelli P, Pasquali S, Cagnoli L, et al: Controlled plasma exchange trial in acute renal failure due to multiple myeloma. Kidney Int 1988;33:1175 [ Links ]
29. Johnson WJ, Kyle RA, Pineda AA, et al: Treatment of renal failure associated with multiple myeloma. Plasmapheresis, hemodialysis, and chemotherapy. Arch Intern Med 1990;150:863 [ Links ]
30. Clark WF, Stewart AK, Rock GA, et al: Plasma exchange when myeloma presents as acute renal failure. Ann Intern Med 2005;143:777 [ Links ]
31. Finkel KW. Is High Cut-Off Hemodialysis Effective in Myeloma Kidney? Seminars in Dialysis 2014; 27:234–236 [ Links ]
32. Kuypers RJ, Lerut E, Claes K, Evenepoel P, Vanrentergham Y. Recurrence of light chain deposit disease after renal allograft transplantation: potential role of rituximab? TransplInt 2007; 20: 381 [ Links ]
33. Uchida S, Matsuda O, Yokota T et al. Adult Fanconi syndrome secondary to k-light chain myeloma: improvement of tubular functions after treatment for myeloma. Nephron 1990; 55: 332 [ Links ]
34. Solak Y, Atalay H, Anil M, et al: Cost of paid transplantation abroad: possible donororigin early multiple myeloma in a renal transplant recipient treated using bortezomib. Transplantation proceedings 2010;42:2813 [ Links ]
Isabel Mesquita
Department of Nephrology, Hospital Curry Cabral
Rua da Beneficência, nº 8
1069-166 Lisboa, Portugal
E-mail: imesquita@sapo.pt
Received for publication: Feb 27, 2016 Accepted in revised form: Aug 29, 2016














