Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.30 no.4 Lisboa dez. 2016
ORIGINAL ARTICLE
Cancer and End-Stage Kidney Disease: A Death Sentence?
Teresa Chuva1, José Maximino1, Joselina Barbosa2, Rui Henrique3,4, Sandra Silva1, Paulo Santos1, Alfredo Loureiro1
1 Department of Nephrology, Portuguese Oncology Institute of Porto, Portugal
2 Department of Medical Education and Biomedical Simulation,Faculty of Medicine of the University of Porto, Portugal
3Department of Pathology, Portuguese Oncology Institute of Porto, Porto, Portugal
4 Department of Pathology and Molecular Immunology, Institute of Biomedical Sciences Abel Salazar – University of Porto (ICBAS-UP), Porto, Portugal
ABSTRACT
Background: End-stage kidney disease is associated with considerable morbidity and mortality, a feature that is shared with malignant neoplasms. Hence, patients with the cumulative effect of these two diseases frequently give rise to the question of whether dialysis should be implemented. The primary goal of this study was to characterize the clinical progression and evaluate the outcome of a group of oncology patients on chronic haemodialysis and also to identify the characteristics associated with prolonged survival. Methods: Retrospective analysis of all patients on the chronic haemodialysis programme in an oncology hospital-based haemodialysis centre between January 1991 and September 2014. Results: 141 patients were treated during this period. The main aetiologies for end-stage kidney disease were multiple myeloma (24.8%) and chronic interstitial disease (22.7%), while the most common tumours were genitourinary cancer (47.5%) and multiple myeloma (24.8%). Multiple tumours were present in 22.0% of patients and 19.2% harboured metastatic disease. Overall, 66.7% of patients died during this period; 7.8% were transferred to other centres as a result of clinical stability; 4.3% recovered renal function; 1.4% received a kidney transplant and 19.9% were still alive at the end of the study. Overall survival was 58.8% at 2 years and 34.8% at 5 years. Multiple myeloma (HR=5.950; 95% CI: 2.512-14.092) and gastrointestinal cancers (HR=3.277; 95% CI: 1.176-9.134) were associated with increased likelihood of death. Conclusions: Survival among patients with often locally advanced or metastatic oncological disease on chronic haemodialysis was unexpectedly high, with 1/3 still alive at 5 years. Accordingly, decision-making in the cancer Key words: cancer, end-stage kidney disease, haemodialysis, prognosis, survival INTRODUCTION End-stage kidney disease (ESKD) is associated with a known burden of morbidity and mortality1, a finding also observed with malignant diseases2. Due to improvements in the management of patients with ESKD on chronic haemodialysis (CHD), survival rates have been increasing, with a consequently growing prevalent population on dialysis3. Meanwhile, significant advances have been made in cancer care, so that even when cure is not possible, many cancers can be controlled and managed for long periods of time, making cancer a chronic condition4. Thus, as an increasing number of elderly patients on CHD are expected to develop cancer as a result of aging5 and, as a consequence of a higher prevalence of cancer survivors overall, the latter are receiving treatment for comorbidities, including chronic kidney disease. In addition, patients on CHD already have a higher risk of developing cancer independently of age6, while oncology patients are particularly prone to developing kidney failure, as a result of the disease or its treatment7,8. Taken together, the combination of cancer and ESKD is an emerging issue in developed countries. Given the dismal prognosis of both entities, nephrologists and oncologists are expected to take challenging decisions regarding renal replacement therapy (starting with the question of whether to initiate treatment or not) and palliative care. However, at present, very little is known about the behaviour and outcome of this particular subset of patients on dialysis. With the aim of improving the quality end-of-life care of patients with both cancer and ESKD, the present study characterized the clinical progression and evaluated the outcome of these patients in an attempt to identify parameters associated with prolonged survival that might aid in clinical decision-making. SUBJECTS AND METHODS Design and Data collection We carried out a retrospective analysis of all patients on a chronic dialysis programme in a single oncology hospital-based haemodialysis center (Instituto Português de Oncologia do Porto, Portugal) between January 1991 and September 2014. Patients defined as being on a chronic dialysis programme included outpatients on dialysis for more than one month. The decision to initiate dialysis was taken by the hospital nephrologists, usually as a result of a multidisciplinary decision shared with the attending oncologists. This was, however, a subjective judgment made on a case-by-case basis, which took into consideration patients performance status and predicted quality of life. The stability of the underlying malignancy was of major importance for the verdict. Metastatic disease did not exclude patients from CHD, except if associated with a terminal disease stage. The collected data included general demographic aspects, such as gender and age, comorbidities (diabetes and hypertension), cause of renal failure, tumour type, presence of metastasis, time on dialysis and outcome (death, transferred to another centre, transplanted or alive on dialysis at the end of the study). Approval to conduct this study was granted by the Ethics Committee (Comissão de Ética para a Saúde) of Instituto Português de Oncologia do Porto, Portugal. Statistical Analysis All analyses were carried out using the Statistical Package for Social Sciences version 22.0 for Windows and R Software version 3.1.0. Overall survival was defined from the time point at which dialysis was started until death from any cause or last known contact. Patients still alive were censored at last follow-up date. Continuous variables were expressed as the median with interquartile range (IQR), and categorical variables were expressed as absolute (N) and relative (%) frequencies. For subsequent analyses, continuous variables were categorized. Differences in demographic and clinical characteristics among groups were tested using the Chi-square test or Fishers exact test for categorical variables and the Mann-Whitney U-test for continuous variables. Bonferronis correction was used for multiple comparisons. Univariate and multivariate analyses were performed by a stepwise backward Cox regression model (p<0.2 was considered as the inclusion criterion for factors that could be added into multivariate analysis). As treatment or diagnostic innovations that can change the survival probability can occur during the follow-up period, we also included a variable in the Cox regression model corresponding to the decade of the patients entry into the study. Hazard ratios are presented together with the 95% confidence intervals (95% CIs). The proportional hazards assumption was checked visually using Schoenfeld residuals. Significance was set at p≤0.05. RESULTS Causes of kidney disease This study was conducted into 141 consecutive patients with a diagnosis of ESKD and cancer, which in most cases was locally advanced or metastatic, that underwent CHD between 1991 and 2014 at our dialysis centre. Figure 1 illustrates the main causes of kidney disease, which included, as most frequent, multiple myeloma (MM) (35; 24.8%) and chronic interstitial nephritis (34; 24.1%), followed by surgical removal of kidney (29; 20.6%).
Table 1 shows demographic and clinical characteristics of the patients, stratified by tumour type. The most common tumours were genitourinary cancer (47.5%) and MM (24.8%) followed by breast (10.6%) and gastrointestinal (8.5%) cancers. Multiple tumours were found in 22.0% of cases. Patients with genitourinary cancer were particularly susceptible, with almost one third (31.3%) with multiple coexistent tumours.
A significant association between tumour type and gender (p=0.001) was depicted, as breast cancers were significantly less prevalent in male patients, as expected.
Finally, radiation enteritis/cystitis was significantly more prevalent in patients with genitourinary cancer (30.2%) (p<0.001), as result of its specific therapeuticstrategy.
Patients baseline characteristics
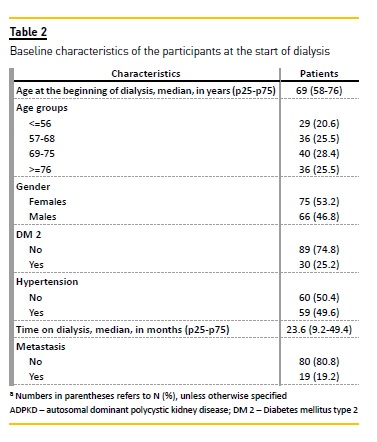
Table 2 presents the baseline demographic and clinical characteristics of the 141 patients at the start of dialysis treatment. The patients age ranged from 20 to 89 years (median=69; IQR=58-76); and 75 (53.2%) of them were female. Almost a fifth of the patients (19; 19.2%) had metastatic cancer. The median time on dialysis was 23.6 months (IQR, 9.2-49.4).
Patients survival and outcomes
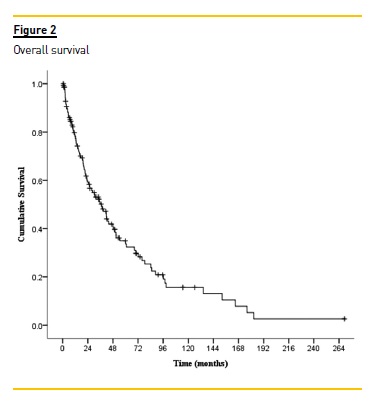
After a median follow-up of 23.6 (IQR: 9.1-49.6) months, 66.7% patients were deceased;, 7.8% were transferred to other centres as a result of clinical stability; 4.3% (all of them with MM) recovered renal function; 1.4% received a kidney transplant and 19.9% were still alive at the end of the study. Globally, survival rates were 58.8% at 2 years and 34.8% at 5 years.
Table 3 shows a Cox proportional hazards regression analysis for progression to death. In univariate analysis, tumour type, gender, age and cause of kidney failure had a p<0.20 on overall survival and were included in the multivariate analysis. In contrast, the presence of metastasis, multiple tumours and the year at the beginning of dialysis were not associated with overall survival.
Finally, the covariates gender and cause of kidney failure were excluded by the backward selection procedure.
According to multivariable analysis, significant predictors of overall survival were tumour type and age. MM (HR=5.950; 95% CI: 2.512-14.092) and gastrointestinal cancers (HR=3.277; 95% CI: 1.176-9.134) were associated with increased likelihood of death.
Furthermore, patients between 57 and 68 years old (HR=2.1; 95% CI: 1.003-4.292); between 69 and 75 (HR=2.720; 95% CI: 1.352-5.473) or over 75 years old (HR=3.118; 95% CI: 1.500-6.480) were at increased risk of dying compared with patients 56 years old or younger.
The causes of death were not possible to determine in 13 out of 94 patients. Of the remaining 81 patients, 64.2% died of tumour progression; 19.8% of cardiovascular events; 4.9% of infection; 8.6% of other causes and 2 patients died of unknown cause.
DISCUSSION
As a result of new therapeutic strategies and improved patient care, there is a growing prevalence of cancer survivors and patients with ESKD on CHD.
Both entities are associated with decreased survival, when compared to the general population1,2 and both represent in themselves a risk factor for each other9-11.
Consequently, it is crucial firstly to recognize the complex relationship between malignancies and chronic renal failure, and secondly to assess eachs impact on the others course of events.
There is, however, a paucity of information concerning the clinical progress and outcomes of oncology patients on chronic dialysis. Thus, our study aimed to characterize a group of oncology patients on CHD followed at a dialysis centre in an oncology hospital. This is, to the authors knowledge, the first report specifically dealing with a group of cancer patients on chronic dialysis for an extended period of time.
In our study, the most common cancers were in the genitourinary system, followed by MM. The former leads to renal impairment mainly through two different mechanisms: either by the development of chronic interstitial nephritis related to urinary tract obstruction12 or surgical kidney loss as a result of treatment strategies13. Enteritis and haemorrhagic cystitis as sequelae of radiotherapy were often found in these patients and frequently coexisted. MM, on the other hand, was the second most prevalent tumour and the first cause of ESKD, mainly as a result of cast nephropathy.
In our dataset, 22% of the patients displayed multiple tumours and these were more common among patients with genitourinary cancer, which may be, at least partially, explained by the multifocal nature of transitional cell carcinoma of the urothelium14,15.
Two patients were considered cured and received a kidney transplant. One was a 62-year-old woman with ovarian cancer and a pheochromocytoma, transplanted after 38 months on dialysis. The other was a woman with an ovarian germ cell tumour who had a first failed renal transplantation at the age of 26 and was finally transplanted with success at age 28 years, after 31 months on dialysis.
Despite the prevalence of MM as a cause of renal injury and its dismal prognosis, patients with MM, were, however, the only subset in which recovery of renal function was observed, as previously reported16,17. The patient who recovered more rapidly was on dialysis for 6 weeks and the one who remained longer on CHD was off haemodialysis after 14 months.
Tumour type was associated with risk of death, with MM and gastrointestinal cancers showing the worst prognosis. This distribution matches that of published reports18 on cancer survival. Interestingly, patients with metastatic disease were not associated with worse survival, probably due to the fact that patients who had metastasis were only admitted for chronic dialysis if considered stable and at low risk of progression in the short-term. Conversely, some patients without metastasis had locally advanced and aggressive tumours, hence not always corresponding to a better prognosis in comparison to those with disseminated disease.
Although, on the one hand, patients treated over the last decade had better treatment options and presumably a better prognosis; on the other hand, patients that began dialysis in our centre more recently have, in general, more comorbidities. Because we are able to provide a better care, many of the patients that are now proposed for CHD would not have even been considered for dialysis two decades ago, including, for instance, many patients with MM. This might explain the lack of statistically significant difference in survival among patients that were treated in 1991 or 2014.
Surprisingly, more than half the patients were still alive after 2 years on haemodialysis and more than one third after 5 years. Most of them died of causes related to the oncological disease, such as complications of chemoor radiotherapy and progressive anorexia and frailty. Interestingly, if we consider that 5-year survival for patients on chronic haemodialysis ranges from 39.8% to 37.2% for diabetic patients19, our data are similar to those described for this subgroup of nononcology patients.
Our results highlight the importance of considering these patients for renal support therapies, demonstrating that a large number of patients might benefit from CHD in spite of the unfavourable prognosis of concomitant cancer and ESKD. There is, however, the need to follow these patients closely and continuously assess their quality of life so that we may offer adequate palliative care, when justified. For that purpose, our unit works in close collaboration with the palliative care team. Patients with disease progression and/or severe decline in quality of life interrupted dialysis after multidisciplinary discussion. If needed, patients were treated with ultrafiltration on an on-demand basis for the relief of dyspnoea.
We could not compare the outcomes of our patient population with other studies, since previous reports usually focused on either patients with renal failure who develop cancer6,20-21 or cancer patients with acute kidney disease22-24. Launay-Vacher et al. has largely contributed to highlighting the risk of renal disease (acute and chronic) in cancer patients25,26, but no data is available for cancer patients on CHD.
There are some limitations to the current study that should be highlighted. Firstly, this is a cohort from a single centre without external validation and, as previously addressed, there may be some bias in the selection of patients for haemodialysis. Secondly, as a retrospective study, patients were not randomly assigned to haemodialysis and selection for CHD was subjective, according to the medical team that assisted the patient.
However, a prospective study regarding this matter would most probably prove unethical. Third, there is the potential for an era-effect. The time period under study was long and spans a time of change in therapy.
To minimize this, we split the group into time periods to compare the outcomes, but no statistically significant differences were disclosed.
CONCLUSION
The paucity of data on cancer patients with concomitant ESKD on CHD makes decisions on whether to initiate or withdraw haemodialysis, as well as prescription of specific cancer therapies, controversial.
We believe that this study sheds a new light on the prognosis of this particular population and indicatesthat a sizeable proportion of these patients may expect a long survival on CHD.
More studies are, however, required to help support clinicians often facing very complex ethical issues.
Studies on quality of life might also usefully explore this matter.
References
1. Tonelli M, Wiebe N, Culleton B et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc of Nephrol 2006; 17: 2034–2047. [ Links ]
2. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. Cancer J Clinicians 2011; 61: 69–90. [ Links ]
3. ESRD in the United States: An Overview of USRDS Annual Data Report Volume 2. Am J Kidney Dis 2015; 66: S79–S92. [ Links ]
4. Phillips JL, Currow DC. Cancer as a chronic disease. Collegian Royal Coll Nursing, Australia 2010; 17: 47–50. [ Links ]
5. Howlader N, Noone AM, Krapcho M GJ et al. SEER Cancer Statistics Review (CSR), 1975-2012 [Internet]. National Cancer Institute2015; Available from: http://seer.cancer.gov/csr/1975_2012/ [ Links ]
6. Stewart JH. Cancers of the kidney and urinary tract in patients on dialysis for end-stage renal disease: analysis of data from the United States, Europe, and Australia and New Zealand. J Am Soc Nephrol 2003; 14: 197–207. [ Links ]
7. Berns JS, Rosner MH. Onco-Nephrology: what the nephrologist needs to know about cancer and the kidney. Clin J Am Soc Nephrol 2012; 7: 1691–1691. [ Links ]
8. Humphreys BD. Onco-Nephrology: kidney disease in the cancer patient: introduction. Semin Nephrol 2010; 30: 531–533. [ Links ]
9. Eneman JD, Philips GK. Cancer management in patients with end-stage renal disease. Oncology 2005; 19: 1199-212-4. [ Links ]
10. Kapoor M, Chan GZ. Malignancy and renal disease. Critical Care Clin 2001; 17: 571–98, viii. [ Links ]
11. Vamvakas S, Bahner U, Heidland A. Cancer in end-stage renal disease: potential factors involved-editorial. Am J Nephrol 1998; 18: 89–95. [ Links ]
12. Coroneos E, Assouad M, Krishnan B, Truong LD. Urinary obstruction causes irreversible renal failure by inducing chronic tubulointerstitial nephritis. Clin Nephrol 1997; 48:125–128. [ Links ]
13. Huang WC, Levey AS, Serio AM et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. The Lancet Oncol 2006; 7: 735–740. [ Links ]
14. Hurwitz M, Spiess P, Garcia J, Pisters L. Urothelial and kidney cancers. Oncology 2014;1–14. [ Links ]
15. Ray P, Sharifi R, Ortolano V, Guinan P. Involvement of the genitourinary system in multiple primary malignant neoplasms: a review. J Clin Oncol 1983; 1: 574–581. [ Links ]
16. Tauro S, Clark FJ, Duncan N, Lipkin G, Richards N, Mahendra P. Recovery of renal function after autologous stem cell transplantation in myeloma patients with end-stage renal failure. Bone Marrow Transplant 2002; 30: 471–473. [ Links ]
17. Lee C-K, Zangari M, Barlogie B et al. Dialysis-dependent renal failure in patients with myeloma can be reversed by high-dose myeloablative therapy and autotransplant. Bone Marrow Transplant 2004; 33: 823–828. [ Links ]
18. American Cancer Society. Cancer Facts and Figures 2015. Atlanta: 2015. [ Links ]
19. ESRD in the United States: An Overview of USRDS Annual Data Report Volume 2. Am J Kidney Dis 2015; 66: S145–S152. [ Links ]
20. Stewart JH, Vajdic CM, van Leeuwen MT et al. The pattern of excess cancer in dialysis and transplantation. Nephrol Dial Transplant 2009; 24: 3225–3231. [ Links ]
21. Wong G, Hayen A, Chapman JR et al. Association of CKD and cancer risk in older people. J Am Soc Nephrol 2009; 20: 1341–1350. [ Links ]
22. Darmon M, Thiery G, Ciroldi M, Porcher R, Schlemmer B, Azoulay É. Should dialysis be offered to cancer patients with acute kidney injury? Intensive Care Med 2007; 33: 765–772. [ Links ]
23. Campbell GA, Hu D, Okusa MD. Acute kidney injury in the cancer patient. Adv Chronic Kidney Dis 2014; 21: 64–71. [ Links ]
24. MacCariello E, Valente C, Nogueira L et al. Outcomes of cancer and non-cancer patients with acute kidney injury and need of renal replacement therapy admitted to general intensive care units. Nephrol Dial Transpl 2011; 26: 537–543. [ Links ]
25. Launay-Vacher V, Chatelut E, Lichtman SM, Wildiers H, Steer C, Aapro M. Renal insufficiency in elderly cancer patients: International Society of Geriatric Oncology clinical practice recommendations. Ann Oncol 2007; 18: 1314–1321. [ Links ]
26. Launay-Vacher V. Epidemiology of chronic kidney disease in cancer patients: Lessons from the IRMA study group. Semin Nephrol 2010; 30: 548–556. [ Links ]
Teresa Rodrigues Chuva, MD
Department of Nephrology
Portuguese Oncology Institute
Rua Dr António Bernardino de Almeida
4200-072 Porto, Portugal
Tel: +351 225084000; Fax: + 351 225084012
Email: m.teresa.chuva@gmail.com
Disclosure of potential conflicts of interest: none declared
Received for publication: Oct 11, 2016
Accepted in revised form: Dec 7, 2016














