Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Portuguese Journal of Nephrology & Hypertension
Print version ISSN 0872-0169
Port J Nephrol Hypert vol.30 no.3 Lisboa Sept. 2016
ORIGINAL ARTICLE
Malignancy after renal transplantation: a single‑centre experience
Pedro Vieira1, Patrícia Barreto2, Sofia Pedroso3, Manuela Almeida3, La Salete Martins3, Leonídio Dias3, António Castro Henriques3, António Cabrita3
1 Nephrology Department, Hospital Central do Funchal
2 Nephrology Department, Hospital de Vila Nova de Gaia
3 Transplant Unit, Nephrology Department, Centro Hospitalar do Porto
ABSTRACT
Introduction: Malignancy management in renal transplant recipients is becoming a major factor affecting long‑term patient survival. Thus, we intended to evaluate both incidence and prognosis of malignant diseases following renal transplantation at a single centre in Portugal. Methods: We studied retrospectively the 2,358 patients who underwent kidney transplantation (KT) between 1983 and 2014. Apart from descriptive analysis, both demographic and clinical characteristics of cancer and non‑cancer cancer patients were compared. Results: During a median follow‑up of 118 (IQR 57‑179) months, 139 patients (5.8%) developed 158 de novo malignancies, with a median time from KT to diagnosis of 76..5 (IQR 21.0‑132.0) months. When compared to non‑cancer patients, they were older at KT date, had longer graft survival and a lower living donor recipients prevalence. As for post‑transplant malignancies analysis, the most common were non‑cutaneous non‑lymphomatous cancers (49.4%, n=78), skin cancers (35.4%, n=56) and post‑transplant lymphoproliferative disorders (9.5%, n=15). Considering specific diagnosis, squamous cell carcinoma and basal cell carcinoma with 17.1% and 16.5% respectively, and non‑Hodgkin lymphomas with 7.6%, were the most frequent. Global mortality among cancer patients was 36.0%, with a median time of 9.7 (IQR 1.9‑17.5) months from time of diagnosis to death. As for survival analysis, cancer patient survival was significantly lower while censored graft survival was significantly higher in this group. Conclusion: Incidence and characteristics of malignancy following renal transplantation in our unit are similar to those globally described, despite some traits probably a result of specific ethnic and environmental characteristics.
Key‑Words: epidemiology; kidney transplantation; neoplasms
INTRODUCTION
Outcomes in kidney transplantation have been improving considerably over the past decades following the introduction of new immunosuppressive drugs, namely in terms of morbidity and mortality from graft dysfunction and failure1. Nonetheless, there are some drawbacks arising from long‑term immunosuppression, such as increased cardiovascular risk and transplant‑related malignancies. For the latter, we must bear in mind that compared to the general population, renal transplant recipients are at a 3 to 5 times higher risk of de novo malignancies, which often assume more aggressive behaviour and worse prognosis2.
Undeniably, malignancy management has become a vital piece of these patients medical follow‑up, as it is fast becoming one of the major factors affecting long‑ term survival3. Thus, underlining the importance of an accurate knowledge of this subject, the present studys focus was on evaluating both incidence and prognosis of malignant diseases following renal transplantation at a single centre in Portugal.
SUBJECTS AND METHODS
This retrospective study identified all patients who underwent kidney transplantation between May 1983 and December 2014 in the Transplant Department of Centro Hospitalar do Porto, Portugal. During this time period, a total of 2,358 kidney transplants were performed, including children (<16 years) and adult patients (≥16 years). The maintenance immunosuppression varied during the follow‑up period as the study covered a large period of time. The regimens comprised azathioprine (Aza) + prednisolone; cyclosporine (CSA) + Aza + prednisolone; CSA vs tacrolimus (FK) + mycophenolate mofetil (MMF) + prednisolone; sirolimus + CSA + prednisolone. Descriptive analysis was used to summarise baseline data as well as clinical data related to de novo malignancies, along with comparison between demographic features and clinical characteristics of cancer versus non‑cancer patients. Kaplan‑Meier analysis was used to estimate overall cumulative patient and graft survivals. All statistical analysis was performed using SPSS software (Statistical Package for the Social Sciences, version 22.0, Evanston, III, United States), considering a p value <.05 as statistically significant.
RESULTS
In our 32‑year experience, a total of 2,358 kidney transplant recipients were kept under follow‑up with a median graft survival of 94.0 (Interquartile range [IQR] 39.0‑149.0) months. Baseline characteristics of the study population are summarised in Table I. Among the total sample, 58.2% were male with a median age of 41.32 (IQR 32.4‑50.2) years at the time of transplantation (with a total of 152 patients being transplanted at a paediatric age). A total of 217 (9.2%) cases involved a living donor and 176 (7.5%) patients received a combined pancreas‑kidney transplant. During the aforementioned period, 139 patients (5.8%) developed de novo malignancies, with a male predominance (n=86, 61.9%) and a median time from transplantation to diagnosis of 76.5 (IQR 21.0‑132.0) months. Among all the patients, 15 (10.8%) presented multiple de novo malignancies.
Statistical comparison between cancer and non‑cancer patients evidenced no significant differences in recipient gender, dialysis vintage, number of kidney transplants, donor age and gender, history of previous acute rejection episodes or greater human leucocyte antigen (HLA) mismatches. Of the characteristics disparities that achieved statistical significance between both cancer and non‑cancer patients, we highlight recipient age, graft survival and donor type, as cancer patients were older at kidney transplantation (KT) date (46.97 [IQR 37.9‑56.1] vs 40.96 [IQR 32.2‑49.6] years, p<.001), had longer graft survival (147,00 [IQR 91.0‑203.0] vs 93,00 [IQR 38.5‑147.5], p<.001) and there was a lower prevalence of living donor recipients (n=2, 1.4% vs n=214, 10.1%, p=.001).
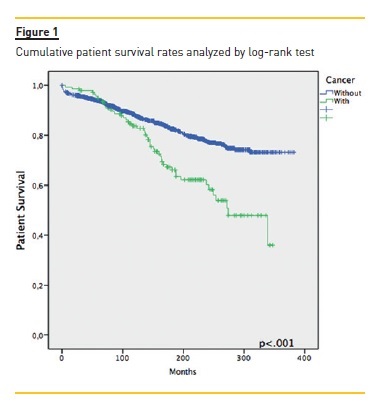
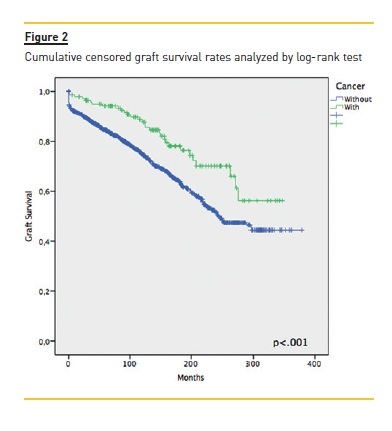
Focusing primarily on post‑transplant malignancies (table II), the most common were non‑cutaneous non‑lymphomatous cancers (49.4%, n=78), herein gathered as a major group; skin cancers (35.4%, n=56); and post‑transplant lymphoproliferative disorders (PTLD) (9.5%, n=15). Focusing on the first group, the three specific tumours that stood out were breast cancer (n= 11, 14.1%), colorectal cancer (n=10, 12.8%) and renal cell carcinoma (RCC) (n=9, 11.5%); while among the skin cancers, squamous cell carcinoma (SCC) and basal cell carcinoma (BCC) with 27 (48.2%) and 26 (46.4%) cases respectively. Of the latter group, non‑Hodgkin lymphomas (NHL) made up 85.7% (n=12). An important remark on two cases (an ovarian adenocarcinoma and a colon adenocarcinoma) where time of diagnosis coincided with that of transplantation, meaning both cancers preceded immunosuppression. The median time between transplantation and malignancy diagnosis was 76.5 (IQR 21.0‑132.0) months, with briefer times among non‑cutaneous non‑lymphomatous cancers (73.0, IQR 20.0‑126.0), Kaposis sarcomas (KS) (20.50 ± 13.1) and those of occult primary cause (6.0 ± 2.8). As for gender distribution, and focusing on malignancies as a whole, there was a clear male predominance almost at a 2:1 ratio. Among skin cancers and PTLD, there was a female predominance (~3:1 and 4:1, respectively), whereas among non‑cutaneous non‑lymphomatous cancers the male/female rates were quite similar. Considering global mortality in the de novo malignancies group, we registered 50 deaths, accounting for 36.0% mortality in this group, though 43 (86.0%) were directly related to malignancy, with a median time of 9.7 (IQR 1.9‑17.5) months between diagnosis and death. As for major subgroup analysis, given its higher inherent mortality, the PTLD group had a 60% mortality (n=9), non‑cutaneous non‑lymphomatous cancers had 37.2% (n=29) while skin cancers were at the opposite end with 3.6% (n=2). The overall cumulative patient and graft survival rates comparing cancer and non‑cancer groups are shown as Kaplan‑Meier curves in Fig. 1 and Fig. 2, respectively.
DISCUSSION
There is cumulative evidence behind the higher risk of de novo malignancies of renal transplant recipients when compared to the general population, though most of the information derives primarily from registries4‑6 and is therefore subject to some bias by way of underreporting, incomplete data on reported malignancies and occasionally inclusion of only first malignancy or exclusion of some types of malignancies7. Nonetheless it is valid information from which one can compare and ascertain subjacent characteristics from the study population.
Thus, when focusing on our data, our incidence of de novo malignancies of 5.8% was similar to other reports from Europe (1% to 9%), the United States (6%) but lower than those from Australia (18 to 28%)8,9.
These numbers reveal an increased incidence when matched to general population, since considering North Region Cancer Registry of Portugal (RORENO)10,11 as a population‑based cancer registry that covers that of our Transplant Units area of influence and comprises data covering most of the follow‑up, we managed to infer a standardized incidence ratio (SIR) of 188 for all cancers except non‑melanoma skin, indicating an 88% increase in these cancers when compared to the general population.
However such excess arises from particular malignancies matching those suggested by the literature findings. Accordingly, when considering specific malignancies we found that skin cancers, except for melanomas, were the most frequent type in KT recipients, followed by PTLDs. The remaining spectrum of cancers was also consistent with the literature data, namely for the high frequency of KS, RCC, anogenital, bladder and hepatocellular carcinomas. Unsurprisingly, virus‑related cancers were frequent, such as NHL associated with infection with Epstein‑Barr virus (EBV) and KS associated with human herpes virus type 8 (HHV‑8) infection. However, two comments must be made. KS had an overall incidence less than 0.3%, in the lower end of multiple studies with incidences ranging from 0.5% for Western and Northern countries to approximately 5% in the Mediterranean regions12, probably a result of low seroprevalence of HHV‑8.
Whereas for PTLD one would expect a shorter time to development (the literatures average of 32 months after transplantation versus the observed mean of 132.3 ± 82.7 months). The incidence of lymphoma development has been reported as highest during the first year after transplantation, when the risk of primary viral infection is highest and the level of immunosuppression is greatest13.
As seen by the Kaplan‑Meier curves, malignancy ostensibly affects long‑term patient survival. However, in the censored graft survival curve, we observed an inversion of the lines, as the subgroup of cancer patients showed a longer graft survival (p<.001), probably arising from the initial difference between both groups as seen by the similar decline rates all throughout follow‑up, consubstantiating the suggestion that longer immunosuppression and follow‑up raise the risk of de novo malignancies and not the way around.
CONCLUSION
As a single‑centre experience, this study supplies valid information with inherent limitations. In a global analysis, overall patient and graft survival rates had markedly good results, yet de novo malignancies following renal transplantation are an important factor affecting long‑term survival, given its greater risk when there is longer immunosuppression. Ultimately, the incidence and characteristics of malignancy following renal transplantation in our unit are similar to those described in other series. However, some of the trends expressed in larger registries are not followed, probably a result of specific ethnic and environment characteristics.
References
1. Briggs JD. Causes of death after renal transplantation. Nephrol Dial Transplant. 2001;16(8):1545‑9. [ Links ]
2. Wong G, Chapman JR. Cancers after renal transplantation. Transplant Rev (Orlando). 2008;22(2):141‑9. [ Links ]
3. Rama I, Grinyo JM. Malignancy after renal transplantation: the role of immunosuppression. Nat Rev Nephrol. 2010;6(9):511‑9. [ Links ]
4. Penn I. Occurrence of cancers in immunosuppressed organ transplant recipients. Clin Transpl. 1998:147‑58. [ Links ]
5. Chapman JR, Sheil AG, Disney AP. Recurrence of cancer after renal transplantation. Transplant Proc. 2001;33(1‑2): 1830‑1. [ Links ]
6. Robson R, Cecka JM, Opelz G, Budde M, Sacks S. Prospective registry‑based Observational cohort study of the long‑term risk of malignancies in renal transplant patients treated with mycophenolate mofetil. Am J Transplant. 2005;5(12):2954‑60. [ Links ]
7. Gutierrez‑Dalmau A, Campistol JM. Immunosuppressive therapy and malignancy in organ transplant recipients: a systematic review. Drugs. 2007;67(8):1167‑98. [ Links ]
8. Besarani D, Cranston D. Urological malignancy after renal transplantation. BJU Int. 2007;100(3):502‑5. [ Links ]
9. Carroll RP, Ramsay HM, Fryer AA, Hawley CM, Nicol DL, Harden PN. Incidence and prediction of nonmelanoma skin cancer post‑renal transplantation: a prospective study in Queensland, Australia. Am J Kidney Dis. 2003;41(3):676‑83. [ Links ]
10. Castro C, Antunes L, Lunet N, Bento MJ. Cancer incidence predictions in the North of Portugal: keeping population‑based cancer registration up to date. Eur J Cancer Prev. 2015. [ Links ]
11. RORENO. North Region Cancer Registry. Porto: Portuguese Oncology Institute of Porto Francisco Gentil – EPE. 2015. [ Links ]
12. S.M. Hosseini‑Moghaddam AS, T. Mazzulli, C. Rotstein, S. Husain. Post renal transplantation Kaposis sarcoma: a review of its epidemiology, pathogenesis, diagnosis, clinical aspects, and therapy. Transpl Infect Dis 2012. 2012;14(4):338‑45.
13. Venyo A A‑HA. Malignancy after Renal transplantation: A review of the literature. Transplantation. 2012;3(3):WMC003186. [ Links ]
Pedro Vieira
Nephrology Department, Hospital Central do Funchal
Avenida Luís de Camões Nº 57, 9004‑514
Funchal, Portugal
Phone: (+351) 291 705 600
Mobile Phone: (+351) 916 535 179
E‑mail: pedro.mds.vieira@gmail.com
Disclosure of Potential Conflicts of Interest: None declared
Received for publication: Mar 20, 2016
Accepted in revised form: July 16, 2016














