Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.29 no.4 Lisboa dez. 2015
ORIGINAL ARTICLE
Early renal protocol biopsies: for some but not for all renal transplant patients?
Biópsias renais protocoladas precoces: uma ferramenta útil em alguns mas não em todos os transplantados renais?
David Navarro, Ana Carina Ferreira, Fernando Caeiro, Patricia Cotovio, Ines Aires, Cecilia Silva, Francisco Remedio, Anibal Ferreira, Helena Viana, Fernanda Carvalho, Fernando Nolasco
Department of Nephrology, Hospital Curry Cabral, Centro Hospitalar Lisboa Central, Lisbon, Portugal.
ABSTRACT
Subclinical rejection following renal transplant is associated with worse outcomes, which can be prevented if recognized early. Protocol allograft biopsies have emerged as an option to identify and allow treatment of subclinical rejection, but optimal timing for their performance is not established.
We retrospectively evaluated a cohort of 52 low immunological risk patients, who were submitted, from 2007 to 2010, to de novo renal transplant. We separated them into two groups depending on performing an early graft protocol biopsy before hospital discharge: Group A – 32 patients (61.5%) performed a protocol biopsy, and group B – 20 patients (38.5%) did not, the biopsy being considered not essential for various reasons. We analysed patients demographics, biopsy complications, graft function, rejection episodes, and patient and graft survival for a median follow-up time of 63.3 months (50.3-83.7).
Group A and group B differed in gender (more female patients were biopsied), dialysis vintage (higher in group A), human leucocyte antigen mismatch (higher in group A), and induction protocol (more patients submitted to thymoglobulin than to basiliximab in group A). Protocol biopsy detected histological changes in four patients (12.5%) in group A (2 cellular and 2 borderline rejections), and all were treated accordingly.
Moderate peri-graft hematoma was reported in two cases (3.9%). Despite the increased risk in group A, renal function at discharge was better than in group B (p < 0.05 for serum creatinine and eGFR). During follow-up, rejection episodes were similar in the two groups. By the end of follow-up (median 63.3 months), proteinuria and renal function were similar between the two groups. Using a multivariate regression model, and despite the initial differences, at the end of follow-up, patients submitted to early protocol biopsies had similar excellent prognosis as the very low-risk patients who were not biopsied. (p = 0.5).
Following our results, we propose that timing of early protocol biopsy should be individualized according to the patients clinical and immunological risk.
Key-Words: Protocol biopsy; renal allograft biopsy; renal transplant; subclinical rejection.
RESUMO
A rejeição subclínica após transplante renal está associada a pior desfecho, que é prevenível se reconhecida precocemente. As biópsias protocoladas surgiram como uma opção para identificar e tratar a rejeição subclínica, embora o timingideal para realização da mesma não esteja estabelecido.
Avaliámos retrospectivamente uma coorte de 52 doentes transplantados renais de novo de 2007 a 2010, com baixo risco clínico e imunológico. Obtivemos 2 grupos baseados na realização ou não de biópsia precoce protocolada do enxerto: Grupo A – 32 doentes (61,5%) foram submetidos a biópsia protocolada previamente à alta hospitalar, e Grupo B – 20 doentes (38,5%) em que a biópsia protocolada foi dispensada, por motivos diversos. Analisámos dados demográficos, complicações da biópsia, função do enxerto, episódios de rejeição e sobrevida do enxerto e dos doentes durante um período de seguimento de 63,3 meses (50,3-83,7).
Os doentes do grupo A e do grupo B eram diferentes no que diz respeito ao género (mais mulheres no grupo A), tempo prévio em diálise (maior no grupo A), human leucocyte antigen mismatch (maior no grupo A) e protocolo de indução (mais doentes submetidos a timoglobulina do que basiliximab no grupo A). As biópsias protocoladas documentaram alterações histológicas em quatro doentes (12,5%) do grupo A (2 rejeições celulares e 2 borderline), todas tratadas. Registaram-se dois hematomas moderados peri renais (3,9% das biópsias). Apesar do risco mais elevado do grupo A, função renal à altura da alta era melhor do que no grupo B (p < 0,05 para creatinina sérica e eGFR). Durante o seguimento dos doentes, o número de rejeições foi idêntico nos dois grupos. No final do seguimento (mediana de 63,3 meses), a proteinúria e a função renal eram semelhantes nos dois grupos. No modelo de regressão multivariada, e apesar das diferenças clínicas iniciais, os doentes submetidos a biópsias protocoladas apresentaram resultados excelentes, semelhantes aos doentes de risco mínimo, não biopsados (p = 0,5).
O momento da realização da biópsia protocolada precoce deve ser ajustado de acordo com o risco clínico e imunológico do doente.
Palavras-Chave: Biópsia enxerto renal; biópsia protocolada; rejeição subclínica; transplante renal.
INTRODUCTION
Even in the current state of the art, acute kidney graft rejection remains a serious risk after transplantation.
Subclinical rejection (SCR) – biopsy-proven graft injury despite normal and stable renal function – has been recognized for more than 30 years. This condition leads to a higher risk of interstitial fibrosis and tubular atrophy (IF/TA)1, and chronic antibody mediated rejection2. Early recognition and treatment of these changes correlates with better graft outcomes3,4.
Such early changes are inefficiently detected by current serum and urinary biomarkers, and are only diagnosed with renal biopsy. Protocol kidney transplant biopsies (PBx) are regarded as a mean to fill this gap and safeguard graft function.
They have proven to be effective in detecting subclinical rejection and are routinely performed in transplantation units worldwide.
Current focus is now on individualizing PBx performance in order to extract maximum benefit from an invasive procedure. For example, patients under an immunosuppression-minimization strategy are at higher risk of rejection and benefit from early (< 3 months) biopsy, while higher risk grafts should be biopsied during the first few weeks. The optimal biopsy timing for patients with a lower immunological risk is still under debate.
In our centre, cadaveric renal transplant patients are submitted to PBx since 2007, and nowadays most of our de novo cadaveric renal transplant (RT) patients undergo a PBx before hospital discharge. In the present study, our primary aim was to compare, in a low immunological risk population, medium-term renal outcome, based on renal function and clinical evolution of those who were submitted to PBx with those who did not, in an attempt to document benefits of very early PBx. Secondary aims were to evaluate the rate of biopsy complications, and patient outcomes as a composite end point of death and graft loss.
SUBJECTS AND METHODS
This is a retrospective descriptive analysis, using data from patient files, of an open cohort of 52 low risk patients submitted to cadaveric de novo renal transplants between January 2007 and December 2010.
Inclusion criteria were: patients submitted to a first renal transplant, with induction protocol based on basiliximab or thymoglobulin (when mismatches were higher than 4), and maintenance immunosuppression based on Tacrolimus (TAC), Mycophenolate Mofetil (MMF) and Prednisolone (PDN).
Exclusion criteria included patients with panel reactive antibody (PRA) superior to 20%, livingdonor transplant, previous renal transplant, multiorgan transplant, patients that died or lost graft function before hospital discharge, and those who had a clinical reason for renal biopsy (e.g., delayed graft function).
Low immunological risk definitions vary widely between transplantation units. We considered patients with a PRA lower than 20%, submitted to singleorgan, first renal transplant. HLA mismatches were not considered, hence the inclusion of patients whose induction protocol included basiliximab or thymoglobulin.
Patients were divided in two groups: those who were discharged after PBx were included in group A, and those who were discharged without PBx were included in group B. Reasons for not proceeding with PBX were mainly logistical (e.g., high influx of patients), and resulted from a positive selection not to perform routine biopsy.
We analysed transplant receptors and organ donors demographic data (age, gender, race), studied period medication (including immunosuppression, antihypertensive medication, statin, allopurinol), time of protocol biopsies, protocol biopsies results (IFTA, presence and type of rejection), protocol biopsies complications, medical complications after transplantation (rejection, new onset diabetes after transplant–NODAT, hypertension, peripheral artery disease), proteinuria at the end of follow-up, graft function and graft and patient survival. Donor-specific antigens (DSA) were reported, when available.
On average, biopsies were performed on the 12th day post-transplant. Biopsy was performed under ultrasound guidance, with a 14-gauge needle, obtaining one core. Biopsies were evaluated in our Renal Morphology Unit, according to the Banff 2007 classification.
Histologic optic examination was performed with haematoxylin and eosin, periodic acid–Schiff, silver and Massons trichrome stains; immunofluorescence study include studies with antibodies against IgG, IgA, IgM, C3 and fibrinogen; immunohistochemistry transplant panel (C4d, SV40) was performed in all biopsies.
Graft function was monitored by serum creatinine and eGFR using the CKD-EPI equation. Tacrolimus trough levels goal was 8-12 ng/ml until month 3 after transplant, and 6-8 ng/ml thereafter. The Hospitals Central Laboratory performed all laboratory measurements.
Data are presented as frequencies for categorical variables; continuous variables are presented as mean ±SD values, when normally distributed, or as median (interquartile range) otherwise.
Comparisons between the 2 studied groups were performed using qui-squared test (for frequencies) and t-test or Wilcoxon for continuous variables, depending on normality. P-values were reported.
After this first analysis, we performed a univariate and a multivariate analysis using all subjects, and defining graft function as our outcome and PBx as our main predictor. In order to do that, and because we were not allowed to perform a linear regression (performing a PBx is not linearly correlated with graft function), we created a new binary variable of graft function, dividing patients according to their final eGFR (> or < to 60 ml/min/1.73m2 – chronic kidney disease stage 3A). We reported p-values, odds ratios (OR) and the 95% confidence interval (95% CI).
All tests were performed using STATA software version 13, and a p-value < 0.05 was considered statistically significant.
RESULTS
From 2007 to 2010, 214 patients were submitted to renal transplant in our transplant unit. Fifty-two patients met the inclusion criteria: 32 patients (61.5%) underwent PBx before hospital discharge – group A – and 20 (38.5%) did not – group B.
The 52 patients were evaluated during a median follow-up of 63.3 months (48.3-98.6). Minimum follow-up was 4 years. Mean age was 52.7 ±12 years, and there were 32 males (61.5%), and 10 diabetics (19.2%). The population had a median haemodialysis vintage of 65.4 months (38.3-120).
Median panel reactive antibody (PRA) was 0%. Donor gender was mostly female (n = 27, 51.9%), average donor age was 47.3 ± 17 years, average human leucocyte antigen (HLA) mismatch of 3.8 ±1.5 and mean cold ischaemia time of 16.8 ± 4.4 hours. Induction protocol was based in Basiliximab in 35 patients (67.3%), and thymoglobulin in 17 patients (32.7%).
As shown in Table I, groups A and B did not differ in age, PRA, presence of DM/NODAT or other comorbidities, cold ischaemia time or donor characteristics.
The 2 groups differed in gender [80% of female patients underwent PBx, versus 50% of male patients (p = 0.03)]; dialysis vintage [75.6 (59-122.2) vs. 38.3 (25.5-103.3) months, p = 0.03)], and HLA mismatch number (Group A 4.16 vs. Group B 3.25, p = 0.03). The majority of patients in the anti-lymphocyte protocol (14/17) did a PBx (p = 0.04). At discharge, serum creatinine was lower in group A (1.3 ± 0.4 vs. 1.6 ± 0.4 mg/dl, p = 0.009), with a trend to higher eGFR (62.1 ± 23.3 vs. 49.8 ± 17.4, p = 0.05). Both groups had similar hospital stay duration. Even so, at the end of follow-up there were no differences in renal function. Nevertheless, patients in group B were followed more time than patients in group A. Using proteinuria as a surrogate marker of graft lesion, there was also no differences between the two groups.
Regarding histology, 32 patients underwent PBx (group A) and sample quality was good, with on average 12 glomeruli (3-26). Histological changes were detected in 4/32 cases (12.5%) – 2 cellular rejections and 2 borderline rejections – all of which were treated with steroids, except in 1 case with thymoglobulin. All biopsies were C4d and SV40 negative. Maintenance immunosuppression remained unchanged in these 4 cases. PBx was complicated by moderate hematoma in 2 cases (3.9%); no other adverse events were reported.
During follow-up, 17 patients developed NODAT (32.7%), 36 were reported as hypertensive (69.2%), seven had peripheral arterial disease (13.7%), 35 dyslipidaemia (67.3%) and nine hyperuricemia (9.6%); 17 patients (33.3%) were under ACEi/ARBs, 29 (57%) with statin and eight (15%) with allopurinol.
During this time, the number of rejection episodes was similar in the two groups: two humoral rejections in group A (1 acute and 1 chronic humoral rejections) and 1 acute cellular rejection in group B.
The two patients in group A who had humoral rejection during follow-up did not have SCR diagnosed on PBx. During follow-up, 38 patients (73%) had one or more anti-HLA screening. A total of eight patients (21%) had a positive donor-specific antigen (with mean fluorescence intensity of 1850 ± 828.1), and an additional 20 patients (38.5%) had a nondonor-specific antigen (mean fluorescence intensity of 2475.2 ± 2186.7). Having a positive anti-HLA positive screening test did not correlate with worse renal outcome.
By the end of follow-up, five patients (9.8%) died – two in group A and three in group B – and two (3.9% – one in each group) lost graft function. The composite end point of death or loss of graft function was not different between the groups (p = 0.3).
Average final serum creatinine was 1.41 ± 0.4 mg/dl, and CKD-EPI eGFR was 56.5 ± 21.8 ml/min/1.73m2. Follow-up details for both groups are summarized in Table II.
After this first analysis, we performed a univariate analysis in order to establish independent predictors of worse renal function in long-term (eGFR < 60 ml/ min/1.73m2). We found the following independent predictors: higher receptors age (p = 0.05), higher donors age (p = 0.01), dialysis vintage (p = 0.04), and eGFR at discharge (p = 0.04). Performing PBx (p = 0.5), was not a predictor of renal function at the end of the study.
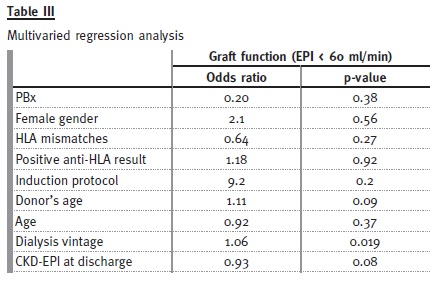
In the multiple regression model (Table III), using PBx, receptors age, donors age, dialysis vintage, eGFR at discharge, gender, anti-HLA test result, HLA mismatch and induction protocol type, we found only dialysis vintage (OR 1.06, 95%CI 1.01-1.11, p = 0.019) to be a significant predictor for worse renal function (eGFR < 60 ml/min/1.73m2) -meaning that for each year on waiting list the odds of worst renal function is 12.7 times higher. There was a trend for significance in donors age (OR 1.11, 95% CI 1-1.25, p = 0.09). Nor PBx, (p = 0.38) not even eGFR at discharge (p = 0.08) were independent predictors of renal outcome.
DISCUSSION
We currently rely on serum creatinine for graft function vigilance, but it is an insensitive biomarker of renal injury, in particular at a GFR of 50-90 ml/ min/1.73m25. Subclinical rejection is associated with worse outcomes and Rush D and co-workers3 have proven that they can be prevented with adequate treatment, in particular if detected at an early stage.
Hence, protocol biopsies, at pre-specified times, have emerged as an appropriate option to detect SCR. We have undertaken a small retrospective study of low immunological risk patients, at a single centre, to compare the outcomes of those submitted to early protocol allograft biopsy versus those who were not.
The PBx have a number of disadvantages that should be considered when engaging in such a programme. Despite being a frequent procedure in transplant units worldwide, allograft biopsies are an invasive procedure with well-documented adverse events. In the era of ultrasound-guided biopsies, major complications are infrequent, happening in 0.4-1.0% of biopsies6,7. Reported complications in our study were somewhat higher (3.9%, two patients); there was no mortality and no graft loss relating to PBx.
Early course biopsies (< 3 month) are useful in detecting SCR, while biopsies performed from 3-12 months additionally identify BK virus nephropathy and CNI toxicity. Findings in PBx after 12 months tend to have a low yield of reversible disease and are not routinely performed5. It is clear that SCR is more frequent in the first few months after RT, but estimates of its prevalence are extremely variable in the literature – 1 to 60%8. This is probably due to variations in timing of biopsy, HLA mismatch and level or type of baseline immunosuppression. The latter is particularly relevant, since modern immunosuppression has led to a decreasing prevalence of early subclinical rejection, with protocols combining MMF and TAC having a notably lower SCR incidence9,10.
This has led to the trial by Rush et al.11 where a lack of benefit for PBx at < 6 months interval was demonstrated in patients under a protocol of MMF, TAC and PDN, probably due to the low incidence of SCR (4.6% in that study).
In our study we compared a group where a decision was taken not to perform the protocol biopsy, due mainly to logistical reasons, and based on the maximum likelihood of excellent clinical prospect (group B), with a normal low risk patients (group A). This was evident on the excellent characteristics of group B when compared to group A, where somewhat higher risk patients were included, as evidenced in Table I.
As expected, we found an important SCR incidence (12.5%) in the PBx group, and all were treated accordingly. Despite the increased risk for group A patients (higher dialysis vintage, more HLA mismatches, more use of thymoglobulin), we did not observe worse outcomes when compared to group B (no biopsy). The number of rejection episodes during FUP was not statistically different in the two groups: 2 humoral rejections in group A and 1 cellular rejection in group B, but again higher risk humoral rejections were only observed in group A.
Despite the association of borderline and SCR rejection with higher incidence of subsequent acute rejection12, no evidence of rejection was present in the early PBx of these two group A patients who developed humoral rejection.
We also found no differences regarding eGFR or proteinuria at end of follow-up. In order to account for these factors, we performed a multivariate analysis, which did not show any differences between these 2 different risk groups.
Ideal timing for PBx is not established, partially because there are other factors in play besides SCR – BK nephropathy and CNI toxicity are the other side of the coin of our current more potent immunosuppression.
Our patients were submitted to PBx, on average, 12 days after transplant, so it is not surprising that we did not observe any episode of BK nephropathy or CNI toxicity.
This study has a number of limitations: it is a retrospective study, with a low number of patients that were submitted to PBx obtaining only one core.
The higher HLA mismatch and dialysis vintage in group A is a clear indication of a positive selection bias towards biopsy for the group foreseen to be of higher risk, despite normal renal function. On the other hand, the group B patients had slightly worse renal function at discharge.
At the end, PBx performed too soon might not be useful in detecting SCR in low clinical or immunological risk patients. As nephrologists, we must balance that with fact that detecting SCR too late might signify we will not be able to reverse its pathological processes, and cannot change outcomes.
Hence, we must personalize PBx timing according to the patients clinical and immunological risk.
CONCLUSION
Protocol kidney transplant biopsies are a well-tolerated procedure, that allow for the diagnosis and treatment of SCR. In this low immunological risk population submitted to very early PBx, 12.5% of patients had evidence of SCR, but we found no changes in the maintenance immunosuppression. Despite having initial markers of a higher risk of worse evolution, the group of patients submitted to early PBx had a similar outcome to the group selected as having a minimal risk transplant, who were not biopsied. Many questions about protocol biopsies remain unanswered, and until then we suggest not performing very early protocol biopsy in minimal risk patients. Trials addressing when and which patients should be submitted to PBx are needed to adequately answer these questions.
References
1. Nankivell BJ, Borrows RJ, Fung CL, OConnell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med 2003;349(24):2326-2333. [ Links ]
2. Moreso F, Carrera M, Goma M, et al. Early subclinical rejection as a risk factor for late chronic humoral rejection. Transplantation 2012;93(1):41-46. [ Links ]
3. Rush D, Nickerson P, Gough J, et al. Beneficial effects of treatment of early subclinical rejection: a randomized study. J Am Soc Nephrol 1998;9(11):2129-2134. [ Links ]
4. Szederkényi E, Iványi B, Morvay Z, et al. Treatment of subclinical injuries detected by protocol biopsy improves the long-term kidney allograft function: a single center prospective randomized clinical trial. Transplant Proc 2011;43(4):1239-1243. [ Links ]
5. Chapman JR. Do protocol transplant biopsies improve kidney transplant outcomes? Curr Opin Nephrol Hypertens 2012;21(6):580-586. [ Links ]
6. Furness PN, Philpott CM, Chorbadjian MT, et al. Protocol biopsy of the stable renal transplant: a multicenter study of methods and complication rates. Transplantation 2003;76(6):969–973. [ Links ]
7. Schwarz A, Gwinner W, Hiss M, Radermacher J, Mengel M, Haller H. Safety and adequacy of renal transplant protocol biopsies. Am J Transplant 2005;5(8)1992–1996. [ Links ]
8. Roberts IS, Stratopoulos C, Zilvetti M, Reddy S, Friend PJ. Impact of immunosuppression on the incidence of early subclinical renal allograft rejection: implications for protocol biopsy policy. Transpl Int 2009;22(8):831-836. [ Links ]
9. Nankivell BJ, Chapman JR. The significance of subclinical rejection and the value of protocol biopsies. Am J Transplant 2006;6(9):2006-2012. [ Links ]
10. Moreso F, Serón D, Carrera M, et al. Baseline immunosuppression is associated with histological findings in early protocol biopsies. Transplantation 2004;78(7):1064-1068. [ Links ]
11. Rush D, Arlen D, Boucher A, et al. Lack of benefit of early protocol biopsies in renal transplant patients receiving TAC and MMF: a randomized study. Am J Transplant 2007;7(11):2538–2545. [ Links ]
12. Choi BS, Shin MJ, Shin SJ, et al. Clinical significance of an early protocol biopsy in living-donor renal transplantation: ten-year experience at a single center. Am J Transplant 2005;5(6):1354-1360. [ Links ]
Dr. David Navarro
Department of Nephrology, Hospital Curry Cabral,
Centro Hospitalar Lisboa Central
Rua da Beneficência, nº8, 1069-166, Lisbon, Portugal
E-mail: davidbnavarro@gmail.com
Conflict of interest statement: None declared.
Received for publication: 02/08/2015
Accepted in revised form: 21/10/2015














