Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.29 no.3 Lisboa set. 2015
CASE REPORT
Henoch-Schönlein purpura associated with pulmonary adenocarcinoma
Púrpura de Henoch-Schönlein associada a adenocarcinoma do pulmão
1Ariana Azevedo, 1Vasco Fernandes, 1David Navarro, 1Ana Carina Ferreira, 1João Sousa, 1Helena Viana, 1Fernanda Carvalho, 1Fernando Nolasco
1Nephrology Department, Centro Hospitalar Lisboa Central, Lisboa, Portugal.
ABSTRACT
Introduction: The Henoch-Schönlein purpura (HSP) is an immunoglobulin A (IgA)-mediated smallvessel systemic vasculitis, rare in adults. The association with solid tumours has been described, especially with lung cancer. Case Report: We present the case of a 60-year-old Caucasian male, diag- nosed with lung adenocarcinoma that underwent surgical resection without (neo)adjuvant theraphy. Two months latter he was admitted for abdominal pain, purpuric rash on his lower extremities and acute kidney injury, with serum creatinine (Scr) of 2 mg/dl. Urinalysis revealed haematuria and 24h proteinuria (P24h) of 1.5 g. The serum protein electrophoresis, complement components C3 and C4, circulating immune complexes, cryoglobulins, ANCA, ANA, anti-dsDNA and the remaining immunologic study as screening for viral infections (HCV, HBV and HIV) were negative. Renal ultrasound was normal and kidney biopsy revealed mild mesangial proliferation; 2 cellular glomerular crescents and 1 fibrinoid necrosis lesion; large amounts of red blood cell casts; lymphocytic infiltration in the intertubular interstitial capillaries; moderate arteriolar hyalinosis. Immunofluorescence demonstrated mesangial and parietal deposits of IgA. The diagnosis of HSP was assumed, and the patient started prednisolone 1 mg/kg/day. Ten months after diagnosis the patients baseline Scr is 1.4 mg/dl with P24h of 0.18g, without haematuria. Conclusion: Although this is a rare association and the exact mechanism behind the disease is yet unknown, physicians should be aware of it. The early recognition and treatment may prevent renal disease progression.
Key-Words: Henoch-Schönlein purpura; lung neoplasms; renal insufficiency; vasculitis.
RESUMO
Introdução: A Púrpura de Henoch-Schönlein (PHS) é uma vasculite sistémica de pequenos vasos associada à deposição de imunoglobulina A (IgA), rara nos adultos. A sua associação com neoplasias sólidas está descrita, sendo a neoplasia do pulmão a mais frequentemente encontrada. Caso Clínico: Apresentamos o caso de um doente de 60 anos, com o diagnóstico de adenocarcinoma do pulmão submetido a ressecção cirúrgica sem terapêutica (neo)adjuvante. Dois meses depois, foi admitido por dor abdominal, púrpura palpável nos membros inferiores e lesão renal aguda, com creatinina plasmática (Pcr) de 2 mg/dl, hematúria e proteinúria/24h (P24h) de 1,5g. A electroforese sérica, C3 e C4, pesquisa de imunocomplexos circulantes e crioglobulinas, ANA, anti-dsDNA e restante estudo imunológico, assim como as serologias virais (VHC, VHB, VIH), foram negativos. A ecografia renal era normal e a biópsia renal revelou glomérulos com discreta proliferação mesangial, 2 glomérulos com crescentes e 1 com necrose fibrinóide; numerosos cilindros hemáticos; presença de linfócitos nos capilares inter-tubulares; moderada hialinose arteriolar. A imunofluo- rescência revelou depósitos mesangiais e parietais de IgA. Admitiu-se PHS e o doente iniciou prednisolona 1 mg/kg/dia, encontrando-se 10 meses após diagnóstico com função renal estabilizada em Pcr 1,4 mg/dl e P24h 0,18g, sem hematúria. Conclusão: Embora a associação entre adenocarcinoma do pulmão e PHS seja rara e o seu mecanismo não esteja esclarecido, devemos estar atentos, dado que o reconhecimento e tratamento atempados podem evitar a progressão da doença renal.
Palavras-Chave: Insuficiência renal; neoplasia do pulmão; púrpura Henoch-Schönlein; vasculite.
INTRODUCTION
Henoch-Schönlein purpura (HSP) is an IgA-medi- ated small-vessel systemic vasculitis with multiorgan involvement, but most notably affects the skin, joints, gastrointestinal tract and kidney1. It is much more common in children but can also occur in adults2. Although the exact cause is presently unknown, malignancy has been reported as a causative factor, with pulmonary, prostatic and renal tumours being the most commonly associated3.
In this report, we present a rare case of HSP associated with lung adenocarcinoma.
CASE REPORT
We present the case of a 60-year-old Caucasian male, with a history of arterial hypertension, since 2013, controlled with bisoprolol, who was a 150 pack-year cigarette smoker. In January 2014, he had a diagnosis of right inferior lobe lung adenocarcinoma (pT2N1M0, without epidermal growth factor receptor gene mutation) and underwent surgical resection without (neo) adjuvant chemoor radiotherapy. Two months later he was admitted to another hospital for abdominal pain. The abdominopelvic CT revealed a diffuse submucosal oedema of the ileum and ascending colon, and the endoscopic study demonstrated multiple mucosal erosions in the same location. The intestinal biopsy was suggestive of a nonspecific inflammatory ileocolic disease. At that time, he developed a purpuric rash on his lower extremities, considered not relevant by a dermatologist, with no need of active treatment. There was no history of arthralgia or arthritis, the patient was not taking any new medication and did not have any known allergies. At this time point, his kidney function was normal (Scr of 0.65 mg/dl) and investigation for haemato-proteinuria was not done. The patient was discharged with the presumptive diagnosis of ischaemic colitis. Two weeks later he was readmitted with per- sistent abdominal pain and referred to our Nephrology Department for acute kidney injury (Scr 2 mg/dl).
On admission, he had no symptoms or signs of arthralgia, arthritis or purpuric rash, he was conscious, oriented, afebrile, blood pressure was 138/73 mmHg, skin and mucous membranes had normal coloration, he was hydrated and anicteric, with normal cardio-pulmonary auscultation and innocent abdominal examination, without peripheral oedema. The remainder of his physical examination was unremarkable.
Initial laboratory testing results revealed abnormal levels of Scr (2.04 mg/dl - eGFR 34 ml/min/1.73m2, calculated by CKD-EPI creatinine equation), urea (84 mg/dl) sodium (132 mEq/L), potassium (5.4 mEq/l) and phosphorous (5.3mg/dl). Serum levels of calcium, uric acid and lactate dehydrogenase were normal. The hae- mogram presented haemoglobin of 9.4 g/dl (normocytic, normochromic), platelet count of 368x10^3mm3, and leukocytes of 11.1x10^9/L (neutrophil 68.4%, lymphocytes 24.3%, monocytes 6.3%, eosinophils 0.6%, baso- phil 0.4%). Urinalysis showed haematuria (erythrocytes 3+/hpf) and proteinuria (150 mg/dL), without epithelial cells or casts and the P24h was 1.5g.
Protein electrophoresis and serum immunoglobulins IgA, IgM and IgG were normal, and there was no evidence of monoclonal component. The subsequent findings were negative or normal for C3 and C4, ANA, anti-dsDNA, ANCA, cryoglobulin, rheumatoid factor, circulating immune complexes, HBsAg, anti-HCV, anti-HIV. Blood and urine cultures were negative.
Renal ultrasound revealed normal sized kidneys with normal parenchymal sinus differentiation.
A renal biopsy was performed and a total of 13 glomeruli were obtained. Two glomeruli showed complete sclerosis due to ischaemia. The remaining glomeruli showed mild mesangial proliferation and in five glomeruli sclerosing proliferative lesions adherent to the Bowman capsule were present. In two of them, cellular glomerular crescents were defined (Fig. 1) and in one a fibrinoid necrosis lesion was present. Generalized tubular atrophy was observed with some tubular necrosis areas and large amounts of red blood cell casts. There was a mild inflammatory infiltrate with lymphocytic infiltration in the intertubular inter- stitial capillaries. There was also moderate arteriolar hyalinosis. Congo red staining was negative, and the immunofluorescence in frozen section demonstrated mesangial and parietal deposits of IgA (Fig. 2) and λ chain (+/++). Skin biopsy was normal.
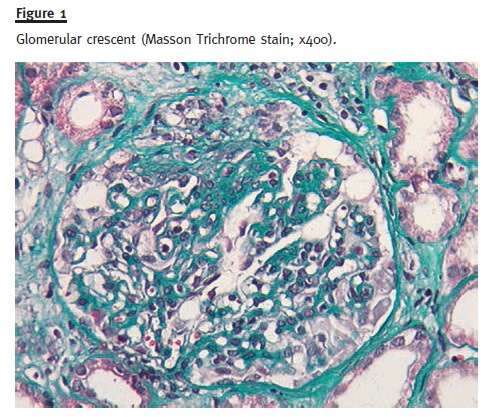
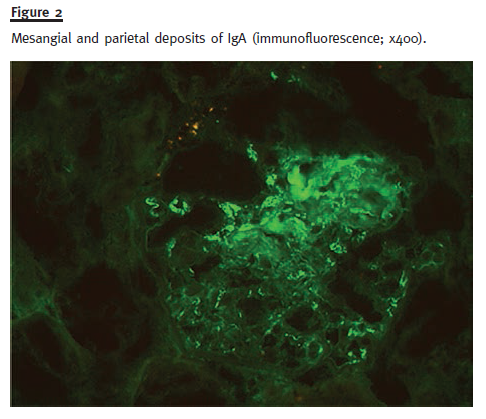
Diagnosis of HSP was made and the patient started prednisolone 1 mg/kg/day (60 mg/day), with no other immunosuppressive agent due to his recent lung cancer diagnosis. At discharge, he was clinically improved. Six months after initiation of prednisolone therapy, tapered to 20 mg/day, he was clinically asymptomatic, with Scr of 1.67 mg/dl and P24h of 0.5 g without evidence of haematuria. Currently, almost one year after, the patient has stabilized kidney function, with Scr of 1.4 mg/dl and P24h of 0.18 g, with prednisolone 2.5 mg/day and without any signs of recurrence of the cancer.
DISCUSSION
Henoch-Schönlein purpura is an IgA-mediated small-vessel systemic vasculitis, which may affect the skin, joints, gastrointestinal tract and kidneys. The likely pathophysiology mechanism behind it involves a process by which the IgA and C3 complexes become deposited on arterioles, capillaries and venules.
The diagnosis of HSP is established on the combination of symptoms, typically characterized by a tetrad of clinical features: purpura, arthralgia, abdom- inal pain and renal impairment. The HSP occurs more often in children than in adults, who frequently develop a more severe clinical syndrome and renal impairment2. Also, the outcome of HSP predominantly depends on the degree of kidney involve- ment4. The diagnostic criteria for HSP, according to the American College of Rheumatology (ACR), are palpable purpura, bowel angina, histological changes of leukocytoclastic vasculitis and age 20 years or younger at onset. The diagnosis of HSP is 81.7% sensitive and 87.7% specific when two or more of these criteria are present5. In our patient, all criteria were present except age, thereby fulfilling the diagnosis of HSP according to the ACR criteria.
Gastrointestinal involvement is frequent in HSP patients, occurring in about two-third of cases, and abdominal pain is nearly constant. In a recent Spanish series, the main clinical manifestations were abdominal pain (100%), nausea and vomiting (14.4%), melena and/or rectorrhagia (12.9%), and positive stool guaiac test (10.3%)6. Abdominal symptoms are possibly secondary to bowel oedema and ischaemia, with descending duodenum and the terminal ileum being the most frequently involved areas, although it may also affect the esophagus, stomach and colon7. Bowel wall thickening with engorgement of mesenteric vessels commonly represents CT scan findings and endoscopic features include diffuse mucosal redness, petechiae, haemorrhagic erosions and ulcers8.
Our patient presented with abdominal pain, and the CT scan showed submucosal oedema of the ileum and ascending colon with an endoscopic study revealing diffuse multiple mucosal erosions in the same location. These findings are compatible with those described for gastrointestinal involvement in HSP patients. Additionally, it was reported that vasculitic endoscopic lesions are documented in only some patients with abdominal symptoms in HSP9. A pos- sible reason for this is that endoscopic biopsies are often superficial and miss the submucosal vessels, thereby missing the vasculitis signs and only revealing nonspecific inflammation7,10. This may explain the finding in our patients intestinal biopsy specimen of nonspecific inflammation without vasculitis.
The aetiology of HSP remains unknown and various triggers have been hypothesized, including bacterial or viral infections, foods, allergens, autoimmune connective tissue disease and drugs11. In the litera- ture, associations between solid malignancies and HSP have been reported in cases where a traditional triggering factor could not be found12. Although haematological malignancies are more frequent than solid tumours in all types of vasculitis, solid tumours are the most commonly associated with HSP and lung cancer has been the most described. As reported by Zurada et al3, 31 patients with HSP had underlying malignancies, among which solid tumours accounted for 61% and lung cancer for 25%. However, no temporal correlations between the onset of the HSP and the cancer diagnosis have been defined.
To the best of our knowledge, only 14 cases of HSP associated with lung cancer are reported to date4. The most common histological diagnoses were squamous cell (n = 8), adenocarcinoma (n = 3) and small cell (n = 3). In the majority of these cases, the onset of HSP was synchronous or preceded the tumour and, less commonly, the HSP symptoms appeared after the diagnosis of lung cancer. In our case, the patient had no correlation with the traditional recognized triggering factors. Onset of HSP occurred 2 months after the lung cancer diagnosis and surgical resection, making it likely that the HSP was associated with the tumour.
The exact correlation between HSP and malignancies remains obscure, but it is possible that the generation and expression of tumour-associated antigen stimulate the immune system to produce aberrant antibodies. These antibodies and antigens react and lead to the formation of immune complexes that can deposit within vessel walls. Further- more, tumour-associated antigens reduce the clearance of circulating immune complexes13,14 and they can also cause lymphocytes dysfunction and lead to the immunoglobulin subtypes shifting from IgM to IgA, which may subsequently cause the release of a large number of aberrant inflammatory cyto- kines, eventualy resulting in vascular inflammation15.
The treatment of malignant tumours may also cause the occurrence of HSP. It is possible that radio- therapy, chemotherapy, and surgical resection change the surface antigens of tumour cells or that tumour-associated antigens are released within the tumour cells following their destruction15. In the present study, the patient developed HSP after surgical resection and although the exact correlation between HSP and lung cancer remains poorly understood, we can postulate that the occurrence of HSP in this patient may be attributed to the malignancy itself, even more on the fact that another triggering factor could not be found.
Treatment for HSP associated with lung cancer has not been well-defined. In some case reports, therapies for lung cancer induced HSP remission, whereas in others steroids were required for HSP improvement, as in our case. We were reluctant to use a more aggressive immunosuppressive treatment attending to the recent lung cancer diagnosis, even though it was seemingly controlled with surgery. Nevertheless, we believe that the corticosteroid therapy contributed to the patients clinical and laboratory improvement.
We report a rare case of HSP associated with pulmonary adenocarcinoma and to the best of our knowledge this is the fourth case described in the literature.
Although this is a rare association and the exact mechanism behind it is yet unknown, physicians should be aware of it. The early recognition and treatment of this condition may prevent the progression of kidney disease to a more advanced stage, where the patients condition and the renal outcome are poorer. It remains unclear what is the optimum treatment for HSP associated with malignancy, but corticosteroid therapy seems to have been beneficial in the clinical management and improvement of our patient.
References
1. Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum 1994; 37(2):187-192. [ Links ]
2. Pillebout E, Thervet E, Hill G, Alberti C, Vanhille P, Nochy D. Henoch-Schönlein purpura in adults: outcome and prognostic factors. J Am Soc Nephrol 2002; 13(5):1271-1278. [ Links ]
3. Zurada JM, Ward KM, Grossman ME. Henoch-Schönlein purpura associated with malignancy in adults. J Am Acad Dermatol 2006; 55(5 Suppl):S65–S70. [ Links ]
4. Zhang XD, Yang SY, Li W, et al. Adult Henoch-Schönlein purpura associated with small cell lung cancer: A case report and review of the literature. Oncol Lett 2013; 5(6):1927-1930. [ Links ]
5. Mills JA, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Henoch-Schönlein purpura. Arthritis Rheum 1990; 33(8):1114-1121. [ Links ]
6. Calvo-Río V, Loricera J, Mata C, et al. Henoch–Schönlein purpura in northern Spain: clinical spectrum of the disease in 417 patients from a single center. Medicine (Balti- more) 2014; 93(2):106-113. [ Links ]
7. Chen MJ, Wang TE, Chang WH, Tsai SJ, Liao WS. Endoscopic findings in a patient with Henoch-Schönlein purpura. World J Gastroenterol 2005;11(15):2354-2356. [ Links ]
8. Esaki M, Matsumoto T, Nakamura S, et al. GI involvement in Henoch–Schönlein purpura. Gastrointest Endosc 2002;56(6):920-923. [ Links ]
9. Novák J, Márki-Zay J, Csiki Z, Sebesi J, Takáts A, Sipka S. [Schönlein-Henoch purpura in adulthood (gastrointestinal manifestation and endoscopy)]. Z Gastroenterol 2001; 39(9):775-782. [ Links ]
10. Kato S, Ebina K, Naganuma H, Sato S, Maisawa S, Nakagawa H. Intestinal IgA deposition in Henoch-Schönlein purpura with severe gastro-intestinal manifestations. Eur J Pediatr 1996;155(2):91-95. [ Links ]
11. Kraft DM, Mckee D, Scott C. Henoch-Schönlein purpura: a review. Am Fam Physician 1998; 58(2):405-408, 411. [ Links ]
12. Mitsui H, Shibagaki N, Kawamura T, Matsue H, Shimada S. A clinical study of Henoch-Schönlein purpura associated with malignancy. J Eur Acad Dermatol Venereol 2009; 23(4):394-401. [ Links ]
13. Sánchez-Guerrero J, Gutiérrez-Ureña S, Vidaller A, Reyes E, Iglesias A, Alarcón-Segovia D. Vasculitis as a paraneoplastic syndrome. Report of 11 cases and review of the lit- erature. J Rheumatol 1990; 17(11):1458-1462. [ Links ]
13. Fortin PR. Vasculitides associated with malignancy. Curr Opin Rheumatol 1996; 8(1):30-33.
14. Hayem G, Gomez MJ, Grossin M, Meyer O, Kahn MF. Systemic vasculitis and epithelioma. A report of three cases with a literature review. Rev Rhum Engl Ed 1997; 64(12): 816-824.
15. Hughes RA, Bottomley DM, Keat AC, Drury A. Henoch-Schönlein purpura occurring in association with carcinoma of the breast. Eur J Med 1993; 2:310-312.
Dra. Ariana Azevedo
Nephrology Department
Centro Hospitalar Lisboa Central
Rua da Beneficência nº8,
1069-166 Lisbon, Portugal.
E-mail: ariana.cerqueira.azevedo@gmail.com
Conflict of interest statement: None declared.
Received for publication: 09/06/2015
Accepted in revised form: 04/09/2015














