Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Portuguese Journal of Nephrology & Hypertension
Print version ISSN 0872-0169
Port J Nephrol Hypert vol.29 no.3 Lisboa Sept. 2015
ORIGINAL ARTICLE
Growing up with chronic renal disease – The road remains rocky
Crescer com doença renal crónica – O desafio continua
Natacha Rodrigues1, Marta Pereira1, Fernando Neves1, Sofia Jorge1, Margarida Almeida2, Rosário Stone2, Gomes da Costa1
1 Department of Nephrology, Centro Hospitalar Lisboa Norte – Hospital Santa Maria, Lisboa, Portugal
2 Department of Paediatric Nephrology, Centro Hospitalar Lisboa Norte – Hospital Santa Maria, Lisboa, Portugal.
ABSTRACT
Background: Understanding the aetiology and approach to management of chronic kidney disease presenting in the paediatric age range continues to represent a true challenge. With the improvement of paediatric care, the number of patients reaching adulthood has increased in recent decades, and it is well known that the transition period from paediatric to adult care is critical; it is associated with a high rate of dropout from care, making it imperative to have an organized system. Methods: Hospital Santa Maria has followed a transition model for the last 17 years in order to optimize medical care for patients with chronic kidney disease who reach adulthood before progressing to end-stage renal disease (ESRD). From 1998 to 2013, our unit has managed 151 such patients. We retrospectively analysed this population with regard to their demographic data, renal diagnosis, follow-up and outcomes. Results: The most prevalent pathologies were uropathies (61 patients), glomerulopathies (46 patients), tubulopathies (18) and cystic diseases (seven), with other causes found in 15 patients. Each group was char- acterized individually. The mean annual decrease of the glomerular filtration rate (GFR) was 2.4ml/ min/1.73m2/year. Fourteen patients developed end-stage renal disease, four were transferred to other hospitals and 18 dropped-out. Eighty-six per cent had finished high school, 60% were working, 32% were students and 8% were unemployed. Conclusion: The aetiology of renal disease presenting in paediatric age is singular. Our dropout rate and annual decrease of GFR are lower than in units with no transition model. The majority of patients choosing haemodialysis had a previous mature fistula. More studies are needed.
Key-Words: Chronic kidney disease; paediatric nephrology; transition.
RESUMO
Introdução: A etiologia, abordagem e manejo da doença renal crónica com início em idade pediátrica constiui um verdadeiro desafio. Nas últimas décadas, com o desenvolvimento dos cuidados pediátricos, o número de doentes a transitar para a nefrologia de adultos tem vindo a aumentar. É do nosso conhecimento que o periodo de transição é uma fase crítica, caracterizada por uma elevada taxa de abandonos, tornando-se deste modo necessário contar com um sistema organizado. Métodos: O Hospital de Santa Maria adoptou um modelo de transição, em vigor nos últimos dezassete anos, de modo a optimizar os cuidados prestados aos doentes renais crónicos pediátricos que atingem a fase adulta sem ainda necessitarem de terapêutica de substituição da função renal. Entre 1998 e 2013, foram observados cento e cinquenta e um doentes na Consulta de Transição. Os autores analisaram retrospectivamente esta população no que respeita a demografia, doença renal, follow-up e outcome. Resultados: As patologias distribuiram-se por uropatias (61 doentes), glomerulopatias (46), tubulopatias (18), doenças quísticas (sete) e outras causas (15 doentes). Cada etiologia foi caracterizada individualmente. A diminuição média anual da taxa de filtração glomerular foi de 2,4 ml/min/1,70m2/ano. Catorze doentes desenvolveram necessidade de terapêutica de substituição da função renal, quatro foram transferidos para outros hospitais e dezoito abandonaram a consulta. Oitenta e seis por cento tinham completado o ensino secundário, 60% estavam empregados, 32% eram estudantes e 8% estavam desempregados. Conclusão: A etiologia da doença renal com apresentação na idade pediátrica é diferente da doença com apresentação na idade adulta. A nossa taxa de abandono, bem como a diminuição média anual da taxa de filtração glomerular são menores do que as registadas por unidades sem modelo de transição implementado. A maioria dos doentes que optou por hemodiálise iniciou técnica por fistula. Mais estudos são necessários nesta área.
Palavras-Chave: Doença renal crónica; nefrologia pediátrica; transição.
INTRODUCTION
The improvement of paediatric renal care in the last decade has resulted in a significant increase in patient survival rates1,2. With earlier detection of urological problems and antenatal screening, a better approach to acute kidney injury and improved management of immunosuppressive therapy, more paediatric patients reach adulthood and transition to adult renal services. It is, therefore, important for hospitals to provide the facilities and processes to make this transition as successful as possible.
The transfer to adult care is accompanied by several challenges not only for the patient and his/her family but also for the nephrologist. The patients face the multiple psychological and physical changes of adolescence, during which they want to be seen as healthy and independent persons, and this may lead to denial and consequent non-adherence to treatment. They also must face a very different system of medical care, with more responsibility and independence, fewer clinic appointments and unfamiliar staff, often taking place in a new organization. Furthermore, children more often develop renal disease from congenital urological abnormalities and hereditary conditions3 than do adults, and adult nephrologists may be less familiar with these conditions than their paediatric counterparts.
In order to reduce the risk of non-adherence at the time of transfer, in 1993 the Society for Adolescent Medicine introduced the concept of transition as a process that involves purposeful, planned efforts to prepare the paediatric patient for the move from a caregiver-directed care to disease self-management in the adult unit4. In 2012, the International Society of Nephrology and the International Pediatric Nephrology Association produced a Consensus Statement5 on the matter, affirming what Professor Cameron presented in his editorial, The rocky road of transition from paediatric to adult care in renal disease, published in this Journal in 20076: adolescents must have developed some competencies before the transfer in order to transit successfully to an adult unit6. At the same time, Dr. Sofia Jorge published in this Journal, Paediatric nephrology patients moving to adult age: a difficult transition to manage. The experience of a paediatric-to-adult out-patient clinic, presenting the data on the first nine years of follow-up after the implementation of a transition model in Hospital Santa Maria7.
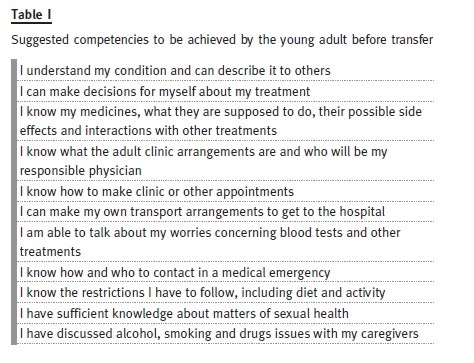
Despite these efforts, there are still no guidelines or universally accepted approaches to this issue. There is also a lack of data on follow-up and out-comes of these patients. Although Professor Paul Harden has published data on transplanted adolescents and their compliance, we found no data or analysis of other transition clinics concerning patients with CKD who have not reached ESRD in the literature and we defend that models of transition should be shared, discussed and validated in order to give the best treatment to our patients.
Therefore, we believe it is of great relevance to share the Hospital Santa Marias retrospective analysis of follow-up and outcomes of our patients.
OBJECTIVES
The aim of this revision is to analyse patients who had at least one visit to our transition unit with regard to the aetiology of kidney disease, follow-up, outcome and demographic data.
SUBJECTS AND METHODS
Since 1998, we have adopted a model where the paediatrician starts the transition process by identifying the adolescents who have successfully acquired the aforementioned expected competencies and referring them to care of the adult unit, where they continue the follow-up. If the patients have reached end-stage renal disease and need renal replacement therapy, they are referred directly by the paediatrician to peritoneal dialysis or trans- plantation units (and are, therefore, not included in our analysis). If not, they are referred to our adult clinic.
The transfer itself initially consists of a first appointment at the adult clinic with the paediatrician, the nephrologist, the patient and the parents all-present. The paediatrician introduces the new doctor, and explains the patients medical condition. The nephrologist then gives information on new rules and the patient is given a choice of whether or not his/her parents will attend future appointments. It is assumed that from that moment, the patient is fully responsible for his/her treatment. Since the introduction of this model, transition meetings have taken place once a week. From the time patients begin adult follow-up, the same nephrologist always sees them.
We retrospectively analysed 151 patients who had at least one visit to our unit with regard to the aetiology of kidney disease, follow-up and outcome. We also characterized the demographics of this population.
RESULTS
One hundred and fifty one patients were transferred to the adult unit: 84 males, 67 females.
Mean age at diagnosis was 6.3 years. Mean age at transfer to adult care was 19.1 years and the mean period of adult follow-up was 5.9 years. Concerning the aetiology of the kidney disease, the most frequent pathologies were uropathies n = 61 (41%), glomerulopathies n = 46 (31%), tubulopathies n = 18 (12%) and cystic diseases n = 7 (5%), with other causes found in 15 patients (10%).
At their first appointment at the adult unit, renal function was normal in 97 patients (71%), with CKD stage 2 in 23 patients (17%), CKD stage 3 in six patients (4%) and CKD stage 4 in 10 patients (7%). The mean annual decrease of GFR was 2.4ml/ min/1.73m2/year. Fourteen patients developed ESRD, four were transferred to other hospitals and 18 (12%) dropped-out, from which, 12 patients dropped-out after five years or more of follow-up.
Of the fourteen patients who developed ESRD, five received kidney transplants, seven started haemodialysis (of which five had already a well-developed arteriovenous fistula) and two started peritoneal dialysis. All patients were previously informed on the different techniques and decided according to their preferences.
Demographic evaluation of 115 patients who currently attend the clinic shows that 12% are married and 11.4% have children. As far as educational attainment is concerned, 86% have finished high school and 4% received special education. Currently, 59.8% are working and 7.8% are unemployed; 52% are students (including full-time and part-time students) and the majority of those (62%) go to college.
Uropathies
Sixty-one patients (40%) were transferred with the diagnosis of an uropathy: 33 were males and 28 were females. Mean age at diagnosis was 2.8 years and mean age at transfer was 18.3 years. Mean duration of follow-up in the adult clinic was 6.2 years. There were eight dropouts. The mean rate of decline of GFR was 2.4ml/min/1.73m2/year and six patients developed ESRD.
This group is subdivided according to aetiology as in the following table:

The Congenital Abnormalities of Kidney and Uri- nary Tract (CAKUT) group comprises 11 patients with renal agenesis, eight with posterior urethral valves, seven with complex congenital uropathy, four with kidney hypoplasia/dysplasia, three with duplex collecting system, and three with pyelo-urethral junction syndrome. There were 11 females and 28 males. We included in this category the syndromal forms of CAKUT represented in our patients by one patient with Beckwith-Wiedemann syndrome, one patient with Rubinstein-Taybi syndrome and one patient with Prune-Belly syndrome.
Uropathy related to repeated infections was observed in 18 patients, only two of which were male. Most of them had borderline criteria for vesicoureteral reflux, so we used as inclusion criteria the absence of any other urologic malformation and a history of repeated infections.
As far as neurologic uropathy is concerned, our four patients had the diagnosis of myelomeningocele resulting in neurogenic bladder.
Glomerulopathies
Forty-six patients (31%) were transferred with a diagnosis of glomerulopathy: 29 males and 17 females.
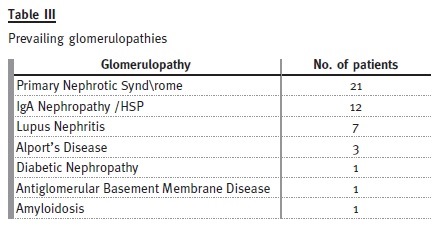
The mean age at diagnosis was 9.2 years and the mean age at transfer was 18.7 years. The mean follow-up time in the adult clinic was 6 years. There were four dropouts and three transfers to other hospitals because of change of address. Regarding renal function, the mean rate of decrease of GFR was 2.6ml/min/1.73m2/year and, during the study period of 15 years, six patients developed ESRD.
Primary nephrotic syndrome (PNS) was the most prevalent cause. Amongst the 21 patients (46%), only one female developed this condition. In this subgroup, 13 patients had relapses, with four of them having corticosteroid resistance. Of those who are still attending our adult clinic, five patients are taking mycophenolate mofetil, three are taking ciclosporin and one is taking tacrolimus. Three patients with PNS developed ESRD, from which two patients were corticosteroid resistant: one had a histological diagnosis of focal segmental glomerulosclerosis and was put on mycophenolate mofetil, the other had two biopsies during his follow-up by the paediatric nephrologist, the first one referring minimal lesion and the second showing focal segmental glomerulosclerosis and he showed no response to cortico- therapy, cyclosporine or mycophenolate mofetil. The third patient presented already with CKD stage 4 at the time of transfer and had an inconclusive kidney biopsy.
Among the 12 patients (26%) who presented with IgA nephropathy/HSP, there was one dropout. In two patients, the renal function deteriorated, with one reaching end-stage renal disease. All the others are in a stable condition, with three patients having occasional episodes of uncomplicated haematuria-proteinuria.
Of the seven patients (15%) who presented with lupus nephritis, two showed progressive decrease in renal function – both with lupus nephritis class IV on biopsy, one reaching ESRD.
Three patients had Alports disease: one dropped out, one developed ESRD and the other has normal GFR.
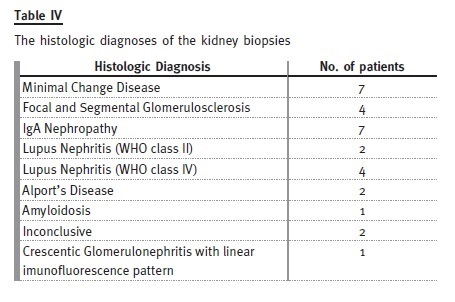
Thirty kidney biopsies were performed in twenty-five patients (19 of the biopsies were performed during paediatric follow-up).
Tubulopathies
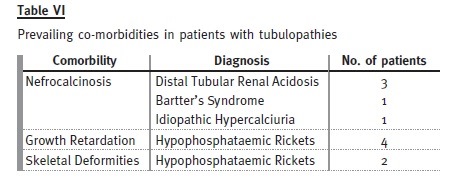
Eighteen patients (12% of the total) were transferred with a diagnosis of tubulopathy: 10 males and eight females.
The mean age at diagnosis was 5.4 years and the mean age at transition date was 22.2 years. Patients have been followed for an average of 25 ± 9 years (6-38 years). Mean rate of decline of GFR was 1.4 ml/ min/1.73m2/year. Only one patient developed ESRD – he had primary hyperoxaluria and presented already a GFR less than 60 ml/min/1.73m2 at the time of transfer. Four patients were lost to follow-up. Only three of the remaining patients had evidence of progression of chronic kidney disease during follow-up after transfer.
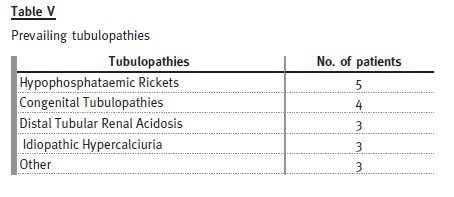
Hypophosphataemic rickets is the most prevalent cause, represented by four females and one male.
Of the patients with distal tubular renal acidosis, all have nephrocalcinosis and one has had several hospital admissions with severe metabolic acidosis and hypokalaemia attributable to non-compliance with therapy. Despite this patient, the others are taking potassium citrate and bicarbonate without major complications.
Congenital tubulopathies are represented by three patients with Bartters syndrome (on indometacin and potassium chloride) and one patient with Gitelmans syndrome (on magnesium supplements and potassium chloride).
These patients already had important co-morbidities at transfer.
Cystic Diseases
Seven patients were transferred with the diagnosis of a cystic disease. Four patients had autosomal dominant polycystic kidney disease (diagnosis made between 12-16 years old; one dropped out and the other three have normal GFR), two patients had tuberous sclerosis and one patient had autosomal recessive polycystic kidney disease (this patient was subsequently transferred to another hospital).
DISCUSSION
Though there are no published data about non- -adherence rates in patients with CKD who have not reached ESRD, there are data available for other chronic diseases. For instance, in a cohort of Cana- dian patients with complex congenital heart defects, published in 2004, only 47% of patients were considered to be successfully transitioned14 In a multicentred randomized controlled Canadian trial, in 2008, the dropout rate was 28% for young adults transferred from paediatric to adult diabetes care15. By comparison, we consider our dropout rate of 12% (18 patients) as a successful outcome. This reflects the work of the committed paediatricians who initiate the transition process and adequately prepare their patients, as well as the organization of our transfer unit; we believe that having the first appointment with paediatrician, nephrologist, patient and family all-present, and taking place in the same hospital, with the same nephrologist that will continue future follow-up, makes a very important contribution. Also, because 12 of the 18 dropouts occurred after at least 5 years of follow-up, we do not believe that they were directly related to transition issues. Unfortunately, we did not have access to the precise number of patients who were actually referred to the first transition appointment and have failed to attend. This lack of information may introduce a bias through selection of those patients who are motivated enough to attend their first appointment.
Analysing and comparing our results with those in Dr. Sofia Jorges paper, in 2007, (where she ana- lysed the transition population of the same hospital at that time)8, we observed a change in prevalence of causes of kidney disease. In her paper, glomerulopathies were the most prevalent aetiology for CKD (28/69). In our study, uropathies were more prevalent (62/151) compared with her results (19/69), suggesting that the approach to this group during childhood might have improved and that they manage to reach adulthood before reaching ESRD more often than in the past.
As expected, patients with uropathies had their diagnoses made earlier in life, followed by tubulopathies and then glomerulopathies (2.8 vs. 5.4 vs. 92 years old). There was no significant difference in gender overall, though there was a difference in some diagnostic categories. Primary nephrotic syndrome was clearly more prevalent in young boys than girls (20:1), with a higher incidence of relapse and response to treatment. Other exceptions were uropathies, where primary vesicoureteral reflux and consequent CKD, occurred as a result of two distinct and gender-related mechanisms: CAKUTs, which were more prevalent in younger males (28:11); and acquired renal scarring which was more frequent in older females (18:2).
There is sparse information about the rate of decline of GFR expected in this population. In KDOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification8, it is stated that the mean rate of decline of GFR for diseases other than diabetes was 2.8-3.9 ml/min/1.73m2. So, we consider our average rate of decline (2.4ml/ min/1.73m2) to be acceptable, probably reflecting fewer comorbidities and better compliance with treatment.
In those patients whose renal disease has progressed to ESRD, we believe that the period of follow-up in our clinic allowed the majority of patients to begin renal replacement therapy with ideal preparation, including the creation of vascular or peritoneal access with adequate time for maturation, and to avoid emergency procedures and their associated risks.
The demographic analysis of social and educational status suggests that, despite having an illness since childhood, our patients are well integrated into society, which is an important holistic perspective to have when assessing outcomes for patients with chronic diseases.
CONCLUSION
More paediatric patients with chronic kidney disease are reaching adulthood before ESRD, bringing the specific aspects of their conditions into the realm of the adult nephrologist. The prevalence of causes and the evolution of CKD are different compared to renal diseases beginning in adulthood.
With only 12% of dropouts among patients going through the transition period, and a mean annual decrease of GFR of 2.4ml/min/1.73m2/year, we believe our model to be successful. Also, the continuity of care allowed the fourteen patients who developed ESRD to be better informed and prepared for renal replacement therapies, with planned creation and time for maturation of an arteriovenous fistula or placement of a peritoneal dialysis catheter, thus avoiding the risks and complications associated with emergency dialysis.
For all the reasons discussed in this paper, we believe that kidney patients in transition to adult care should be followed by a specific team who can devote particular care to them during this important period.
References
1. Lewis MA, Shaw J, Sinha M, et al. Demography of the UK paediatric renal replacement therapy population. In: UK Renal Registry Report Bristol, UK: UK Renal Registry 2008; Chapter 13:257–267.
2. Ferris ME, Gipson DS, Kimmel PL, Eggers PW. Trends in treatment and outcomes of survival of adolescents initiating end-stage renal disease care in the United States of America. Pediatr Nephrol 2006;21(7):1020–1026. [ Links ]
3. Ferris ME, Mahan JD. Pediatric chronic kidney disease and the process of health care transition. Semin Nephrol 2009;29:435–444. [ Links ]
4. Blum RW, Garell D, Hodgman CH, et al. Transition from child-centered to adult health-care systems for adolescents with chronic conditions. A position paper of the Society for Adolescent Medicine. J Adolesc Health 1993;14(7):570–576. [ Links ]
5. Watson AR, Harden PN, Ferris M, Kerr PG, Mahan JD, Ramzy MF. Transition from pediatric to adult renal services: – A consensus statement by the International Society of Nephrology (ISN) and the International Pediatric Nephrology Association (IPNA). Kidney Int 2011;80(7):704-707. [ Links ]
6. Cameron JS. The rocky road of transition from pediatric to adult care in renal disease. Port J Nephrol Hypert 2007;21(3):185-186. [ Links ]
7. Jorge S, Neves FC, Mendonça E, Stone R, Almeida M, Prata MM. Paediatric nephrology patients moving to adult age: a difficult transition to manage. The experience of a paediatric-to-adult out-patient clinic. Port J Nephrol Hypert 2007;21(3):211-217. [ Links ]
8. National Kidney Foundation. K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification and Stratification. Am J Kidney Dis 2002; 39(2 Suppl 1):S1-S266. [ Links ]
9. Watson AR. Problems and pitfalls of transition from paediatric to adult renal care. Pediatr Nephrol 2005;20(2):113–117. [ Links ]
10. Viner RM. Transition of care from paediatric to adult services: one part of improved health services for adolescents. Arch Dis Child 2008;93(2):160–163. [ Links ]
11. Betz CL. Approaches to transition in other chronic illnesses and conditions. Pediatr Clin North Am 2010;57(4):983–996. [ Links ]
12. Blum RW, Garell D, Hodgman CH, et al. Transition from child-centered to adult health-care systems for adolescents with chronic conditions. A position paper of the Society for Adolescent Medicine. J Adolesc Health 1993;14(7): 570–576. [ Links ]
13. Ferris ME, Wood D, Ferris MT, et al. Toward evidence-based health care transition: The Health Care Transition Research Consortium. Int J Child Adolesc Health 2011;3(4):479–486. [ Links ]
14. Reid GJ, Irvine MJ, McCrindle BW, et al. Pevalence and correlates of successful transfer from pediatric to adult health care among a cohort of young adults with complex congenital heart defects. Pediatrics. 2004;113(3 Pt 1):e197-205. [ Links ]
15. Spaic T, Mahon JL, Hramiak I, et al. with the JDRF Canadian Clinical Trial CCTN1102 Study Group. Multicentre randomized controlled trial of structured transition on diabetes care management compared to standard diabetes care in adolescents and young adults with type 1 diabetes (Transition Trial). BMC Pediatr 2013;13:163. [ Links ]
Dra Natacha Rodrigues
Department of Nephrology
Centro Hospitalar Lisboa Norte – Hospital Santa Maria
Av. Prof. Egas Moniz
1649-035 Lisboa, Portugal
Email: rodrigues120@hotmail.com
ACKNOWLEDGMENTS
The authors would like to express their respect and acknowledgement to Professor Fernando Coelho Rosa, pioneer of Paediatric Nephrology in Portugal. A thankful thought to the other paediatricians of the paediatric nephrology clinic, namely, Dr. José Silva and Dr. Carla Simão.
Conflict of interest statement: None declared.
Received for publication: 14/07/2015
Accepted in revised form: 28/08/2015














