Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.29 no.3 Lisboa set. 2015
REVIEW ARTICLE
Benefits of selective vitamin D receptor activators in kidney transplanted patients
Benefícios dos ativadores seletivos dos recetores de vitamina D em doentes transplantados renais
Aníbal Ferreira1,2, Inês Aires1,2, Fernando Nolasco1,2, Domingos Machado3,4,Fernando Macário5, Pedro L. Neves6,7, António G. Costa8, António M. N. Cabrita9,10, Rui Castro11, João B. Pereira12
1 Nephrology Unit, Centro Hospitalar Lisboa Central EPE, Hospital Curry Cabral, Lisbon, Portugal
2 Nova Medical School, Faculdade de Ciências Médicas da Universidade Nova de Lisboa, Lisbon, Portugal
3 Transplantation Unit, Centro Hospitalar de Lisbo Ocidental EPE, Hospital de Santa Cruz, Carnaxide, Portugal 4 Portuguese Society of Diabetology, Lisbon, Portugal
5 Nephrology Unit, Centro Hospitalar e Universitário de Coimbra EPE, Coimbra, Portugal
6 Nephrology Unit, Centro Hospitalar do Algarve EPE, Faro, Portugal
7 Department of Biomedical Sciences and Medicine, Universidade do Algarve, Faro, Portugal
8 Nephrology Unit, Hospital de Santa Maria, Centro Hospitalar Lisboa Norte EPE, Lisbon, Portugal
9 Nephrology Unit, Centro Hospitalar do Porto EPE, Porto, Portugal
10 Uninefro Matosinhos, Matosinhos, Portugal
11 Nephrology Unit, Centro Hospitalar de Trás-os-Montes e Alto Douro EPE, Vila Real, Portugal
12 Scientific ToolBox Consulting, Lisbon, Portugal.
ABSTRACT
Severe chronic kidney disease may lead to disturbances, such as hyperphosphatemia, increased secretion of fibroblast growth factor-23 (FGF-23) and vitamin D deficiency. These may increase plasmatic levels of parathyroid hormone, and decrease plasmatic levels of calcium. Altogether, these may contribute to the development of secondary hyperparathyroidism, and to abnormalities in mineral metabolism. Kidney transplantation is the best option to improve longevity and quality of life in end-stage chronic kidney disease patients. Vitamin D deficiency has been associated with cardiovascular disease, which is the leading cause of death in chronic kidney disease. Therefore, diagnosing this deficiency may be pivotal for minimizing mortality in chronic kidney disease, because pharmacological treatments for this deficiency may be prescribed. Calcitriol is indicated for the treatment of vitamin D deficiency, both in chronic kidney disease and in kidney transplanted patients. However, calcitriol may increase the plasmatic levels of calcium and phosphorous, which can lead to vascular calcifications, that have been associated with cardiovascular mortality. Selective vitamin D receptor activators are indicated for the treatment of vitamin D deficiency in chronic kidney disease. These have the advantage of being associated with lower increases of plasmatic levels of calcium and phosphorous. These drugs also seem to have additional effects that may minimise patient morbidity and mortality, especially due to potentially reducing cardiovascular events. Unfortunately, there are few studies about the use of these drugs in kidney transplanted patients. Here we present a review about the physiology of vitamin D, the consequences of its deficiency in chronic kidney disease and in kidney transplanted patients, and about the diagnosis and treatment of this deficiency. Finally, we discuss the new line of research about the efficacy and safety of selective vitamin D receptor activators in kidney transplanted patients.
Key-Words: Calcitriol; cholecalciferol; CRF-chronic renal failure; paricalcitol; renal Insufficiency, chronic; review; vitamin D.
RESUMO
A doença renal crónica pode conduzir a distúrbios metabólicos como hiperfosfatemia, aumento da secre- ção do fibroblast growth factor- 23, e deficiência de vitamina D, que por sua vez, podem conduzir a um aumento dos níveis plasmáticos da paratormona, e a uma diminuição dos níveis plasmáticos de cálcio. Consequentemente, a doença renal crónica pode contribuir para o desenvolvimento de hiperparatiroidismo secundário, e de anomalias no metabolismo mineral ósseo. A transplantação renal é a opção que proporciona a maior longevidade e qualidade de vida, a doentes com doença renal crónica terminal. A deficiência de vitamina D tem sido associada a doença cardiovascular, que é a causa principal de mortalidade nos transplantados renais. Logo, o diagnóstico desta deficiência poderá ser crucial para tentar minimizar esta mortalidade, uma vez que esta deficiência de vitamina D pode ser corrigida terapeuticamente. O calcitriol tem como indicação terapêutica o tratamento da deficiência de vitamina D, tanto em doentes com doença renal crónica, como em doentes transplantados renais. No entanto, este fármaco pode aumentar os níveis plasmáticos de cálcio e de fósforo, conduzindo a potenciais calcificações vasculares, que têm sido associadas a mortalidade cardiovascular. Os ativadores seletivos dos recetores da vitamina D têm como indicação terapêutica o tratamento da deficiência de vitamina D na doença renal crónica. A vantagem destes fármacos é que têm sido associados a aumentos inferiores dos níveis plasmáticos de cálcio e fósforo. Estes fármacos têm ainda efeitos adicionais que podem minimizar a morbilidade e a mortalidade, principalmente devido ao seu potencial para reduzir eventos cardiovasculares. Neste artigo apresentamos uma revisão de literatura sobre a fisiologia da vitamina D, sobre as consequências da sua deficiência na doença renal crónica e em doentes transplantados renais, e sobre o diagnóstico e tratamento desta deficiência. Finalmente, discutimos os resultados de estudos recentes, sobre a segurança e eficácia dos ativadores seletivos dos recetores da vitamina D em doentes transplantados renais.
Palavras-Chave:Calcitriol; colecalciferol; insuficiência renal, crónica; paricalcitol; revisão; vitamina D.
INTRODUCTION
Disturbances in bone and mineral metabolism may be observed since early stages of chronic kidney disease (CKD). For example, mild CKD (or stage 2 CKD), is characterised by an estimated glomerular filtration rate (GFR) between 60 and 90 ml/min/1.73 m2, as well as by disturbances in bone and mineral metabolism, such as increased levels of fibroblast growth factor-23 (FGF-23), and decreased levels of calcidiol and calcitriol. Usually, at these stages of CKD, serum phosphate levels are either normal or reduced, due to the effects of the phosphaturic hormones (FGF-23 and parathyroid hormone [PTH]) on the kid- ney tissue that still responds to these, and also due to the decrease in intestinal phosphate absorption (consequence of the vitamin D deficiency)
More severe CKD, such as stage 5 CKD, is characterized by a GFR < 15 mL/min/1.73 m2 and, if untreated, by severe disturbances in bone and mineral metabolism, such as hyperphosphatemia, increase in FGF-23, and active vitamin D deficiency, due to a decreased activity of the 1α-hydroxylase (an enzyme mainly present on the tubular cells of the kidney, that hydroxylates 25-OH-vitamin D)1. This leads to the rise of the plasmatic levels of PTH and eventually to hypocalcaemia. Altogether, these contribute to the development of secondary hyperparathyroidism (SHPT) and to abnormalities in mineral metabolism.
Kidney transplantation is the treatment option that offers the best longevity and quality of life for end-stage CKD patients. However, although successful kidney transplantation may improve GFR, kidney transplanted patients may still have CKD, which may lead to severe mineral bone metabolic disease. Furthermore, despite a better 1α-hydroxylase activity, kidney transplanted patients may still have vitamin D deficiency for several reasons, such as: inadequately high FGF-23 serum levels, regular use of corticosteroids (part of the normal post-transplant immunosuppressive therapy), low exposure to sun light (normally recommended to prevent cutaneous tumours), diet restrictions, and mild to moderately decreased kidney function always present after kidney transplant.
Vitamin D deficiency has been associated with cardiovascular disease (CVD), which is the leading cause of death in CKD2. There is no clear evidence from randomised controlled trials that vitamin D supplementation may be associated with a reduction of mortality in CKD patients. However, according to a meta-analysis by Duranton et al.3 that included 14 observational studies (observing altogether more than 35,155 deaths, in a total population of 194,932 patients with CKD or undergoing dialysis), supplementation with vitamin D derivatives is significantly associated with a 27% relative risk reduction of all-cause mortality (relative risk 0.73, 95% CI 0.65–0.82) and with 37% relative reduction of cardiovascular mortality risk (relative risk 0.63, 95% CI 0.44–0.92). Therefore, diagnosing vitamin D deficiency in CKD may be pivotal for minimizing mortality of CKD patients, because appropriate pharmacological treatments may be pre- scribed for this deficiency.
Calcitriol has been prescribed to treat vitamin D deficiency both in CKD and in kidney transplanted patients4. Although calcitriol may treat this deficiency, undesirably it also may increase the plasmatic levels of calcium and phosphorous, which can lead to vas- cular calcifications that have been associated with mortality5-7.
Alternatively, selective vitamin D receptor activators (VDRAs) may also be used to treat vitamin D deficiency in CKD, with the advantage of being associated with a lower incidence of hypercalcaemia and hyperphosphatemia8-10. Furthermore, these drugs also seem to have non-inflammatory (including immunomodulatory) and anti-inflammatory effects, which may minimise patient morbidity and mortality, especially due to potentially reducing cardiovascular events11-13.
Given the benefits of paricalcitol in CKD (the only selective VDRA drug currently licensed in Europe and USA), it is possible to hypothesise that this drug may also benefit kidney transplanted patients. Unfortunately, there are few studies investigating this.
In this review we present an overview about the physiology of vitamin D, the consequences of its deficiency in CKD, especially after kidney transplantation. Furthermore, we discuss the state of the art about the treatment of vitamin D deficiency in CKD, including the non-inflammatory (including immunomodulatory) and anti-inflammatory benefits of selective VDRA therapy. Finally, we discuss the current line of research about the efficacy and safety of selective VDRAs in kidney transplanted patients.
THE PHYSIOLOGY OF VITAMIN D
Vitamin D is a steroid-derived hormone whose activation requires several enzymatic steps. Briefly, 7-dehydrocholesterol is photo-activated in the skin into cholecalciferol, which is then converted in the liver into calcidiol (25[OH]D), which is finally converted in the kidney (by the 1α-hydroxylase) into calcitriol (1,25[OH]2D3) that is the most active form of vitamin D. Then calcitriol binds to nuclear vitamin D receptors (VDRs), regulating the expression of more than 200 genes, such as those encoding the FGF-23 and its co-receptor klotho. These play a major role in the regulation of plasmatic calcium and phosphorous
Vitamin D deficiency has been associated with opportunistic infections after kidney transplantation14, and cancer15, and it is not yet clear whether vitamin D supplementation may prevent these16, 17. Furthermore, as presented below, this deficiency may also lead to SHPT, CVD, and to poor outcomes after kidney transplantation.VITAMIN D DEFICIENCY AND SECONDARY HYPERPARATHYROIDISM
Secondary hyperparathyroidism is a common metabolic disorder that may occur due to vitamin D deficiency, both in CKD18 and in post-kidney transplantation patients19. This disorder is characterized by an increased synthesis of PTH by the parathyroid gland, contributing to bone mass loss20 and hypercalcaemia21. Therefore, the reduction of the plasmatic levels of PTH toward normal levels is a strategy for preventing or treating SHPT. As it will be presented in detail later in this review, this can be done using drug classes such calcimimetics, non-selective VDRAs, and selective VDRAs. Treating SHPT is important because this disease has been associated with CVD, due to ventricular hypertrophy and coronary heart disease22.
VITAMIN D DEFICIENCY AND CARDIOVASCULAR DISEASE
Vitamin D deficiency has been associated with CVD morbidity and mortality. A recent cohort study23 investigated the association between pre-operative plasmatic levels of calcidiol (a precursor of vitamin D) and major cardiac and cerebral vascular events in a sample of 4,418 cardiac surgical patients. For 38.0% of patients these levels were deficient (i.e., < 30 nmol/L), for 32.3% insufficient (i.e., between 30 and 49.9 nmol/L), and for 3.1% high (i.e., > 100 nmol/L). The odds ratio for major cardiac and cerebrovascular events was 2.23 (95% CI, 1.31-3.79) for deficient levels, 1.73 (95% CI 1.01- -2.96) for insufficient levels, and 2.34 (95% CI, 1.12-4.89) for high levels, when compared to calcidiol levels between 75-100 nmol/L. Therefore, it was concluded that vitamin D deficiency is prevalent in pre-operative cardiac surgical patients, and that it is an independent risk factor for major cardiac and cerebral vascular events. Further research is still needed to investigate whether native vitamin D supplementation may reduce the risk of CVD in CKD patients24.
THE ROLE OF VITAMIN D DEFICIENCY IN THE OUTCOME OF KIDNEY TRANSPLANTATION
Vitamin D deficiency has been associated with a poor outcome of renal transplantation, but it is unclear whether this may be improved by correcting this deficiency. For example, a retrospective study25 suggested that vitamin D supplementa- tion, between 3 and 12 months post-transplantation, did not confer structural and functional nephro-protection in a sample of 64 patients. However, a recent study26 suggested that vitamin D supplementation, within the first 90 days post-transplantation, was associated with fewer cases of acute cellular rejection. Further research is still needed to investigate whether vitamin D supple- mentation may improve the outcome of kidney transplantation.
For all the above, the diagnosis of vitamin D deficiency in CKD, and in post-kidney transplantation, may be pivotal for deciding on the appropriate- ness of prescribing a treatment for it. This may potentially prevent not only SHPT and CVD, but also poor kidney transplantation outcomes, reducing morbidity and mortality of CKD patients.
DIAGNOSIS OF VITAMIN D DEFICIENCY
Vitamin D deficiency is diagnosed by dosing deficient plasmatic levels of calcidiol. However, there is no consensus regarding its normal and pathological plasmatic levels. Generally, plasmatic levels of calcidiol between 30 and 150 ng/ml (75-375 nmol/l) are indicative of normal vitamin D levels, above 150 ng/ ml (375 nmol/l) are indicative of excessive vitamin D levels, between 20 and 30 ng/ml (50-75 nmol/l) are indicative of deficient vitamin D levels, and below 20 ng/ml (50 nmol/l) are indicative of severely deficient vitamin D levels2, 27.
TREATMENT OF VITAMIN D DEFICIENCY IN SECONDARY HYPERPARATHYROIDISM
Secondary hyperparathyroidism may be treated with calcimimetic drugs (e.g., cinacalcet), and/or with vitamin D receptor activators (VDRAs), which may be non-selective (e.g., alfacalcidiol, calcitriol) or selective (e.g., paricalcitol)10,28. Cinacalcet can induce hypocalcaemia and, therefore, it requires frequent monitorization of the plasmatic levels of calcium. Calcitriol has been used to treat SHPT in CKD patients, because it binds to VDRs in the parathyroid glands reducing PTH production29. It also acts in the intestines increasing calcium absorption, and in the bones balancing their reabsorption and formation. Unfortunately, this treatment has important side effects such as hypercalcaemia and hyperphosphatemia, which may increase the risk of extraosseous calcifications that have been associated with increased cardiovascular mortality6.
Unlike with non-selective VDRAs, selective VDRAs are more specific to VDRs located in the parathyroid, rather than those located in the intestines and bone. Therefore, the action of selective VDRAs results in lower increases of the plasmatic levels of calcium and phosphate8, 9. Paricalcitol is the only commercially available selective VDRA in Europe and USA, and it is licensed for the prevention and treatment of SHPT in CKD patients (stages 3, 4, and 5), both in pre-dialysis and during dialysis.
Paricalcitol reduces PTH levels, with fewer cases of hypercalcemia and hyperphosphatemia, and prevents deleterious bone reabsorption. According to an early double blind randomised controlled trial9, paricalcitol decreased PTH plasmatic levels in CKD patients with SHPT, by approximately 60% over 12 weeks of study, and it led neither to hyper- calcaemia, nor to significant increases in plasmatic phosphate levels. Furthermore, a recent prospective observational study8 investigated the safety and efficacy of intravenous paricalcitol in the treatment of SHPT in 1,313 dialysed patients. After 6 months of treatment, a PTH reduction of ≥ 30% was observed in 63.0% of the patients, and a PTH reduction of ≥ 60 % was observed in 35.9% of patients. Furthermore, calcium and phosphorous levels remained stable over time in the majority of patients, and alkaline phosphatase (i.e., a bone turn-over marker) plasmatic levels improved signifi- cantly (p < 0.0001) from baseline (median = 98U/L) to study end (median = 83 U/L).
Further research is still needed to compare all- -cause mortality, CVD morbidity, and CVD mortality, for SHPT patients treated with paricalcitol versus cinacalcet24. In a recent RCT30, the use of paricalcitol was associated with beneficial effects on bone turn- over markers (alkaline phosphatase and bone specific alkaline phosphatase) when compared to cinacalcet. However, levels of FGF-23 were increased with paricalcitol, and this could suggest that paricalcitol was associated with a higher risk of death than cinacalcet. This result may be explained by the different mechanisms of action of these drugs. Cinacalcet reduces PTH plasmatic levels by antagonizing calcium-sensing receptors, whereas paricalcitol does this by activating VDRs, which may also promote the production of FGF-23. The authors of this study sug- gested that it is necessary to further investigate the association between FGF-23 and death, because paricalcitol has been consistently associated with decreased mortality in numerous studies31-37.
Observational studies suggest that the risk of death (from all causes) in CKD patients is significantly reduced with paricalcitol versus calcitriol, or no treatment. For example, a cohort study37 investigated survival in 67,399 patients on haemodialysis, taking either paricalcitol (n = 29,021) or calcitriol (n = 38,378). It suggested that patients taking paricalcitol had 16% (95% CI; 10-21) superior 3-year survival rate than those taking calcitriol. Increased survival rates were also identified for patients taking paricalcitol versus no treatment31-35, but survival rates for paricalcitol (15.3 [95% CI; 13.6-16.9] deaths/100 patient-years) versus doxercalciferol (15.4 [95% CI; 13.6-17.1] deaths/100 patient-years) were not found to be significantly different33.
Studies have also reported that paricalcitol is associated with lower CVD mortality, when compared to calcitriol. Indeed, it was reported that the CVD death rates for patients taking paricalcitol were 0.106 per person-year, whereas for those taking calcitriol were 0.128 per person-year37. Similarly, lower death rates due to CVD were also identified for treatment with alfacalcidiol when compared to no treatment36. These results are encouraging, because it is known that CVD is a major cause of death in CKD patients.
Curiously, studies report that CKD patients taking paricalcitol have lower mortality rates, independently of the plasmatic levels of calcium, phosphorous, or PTH at the end of study35, 37. This suggests that the benefits of paricalcitol may not be limited to its effects on mineral and PTH metabolism. In fact, as presented below, several studies proposed different potential non-inflammatory (including immunomodu- latory) and anti-inflammatory effects for paricalcitol (or selective VDRAs) that may explain its improved survival rates in CKD patients5.
NON-INFLAMMATORY AND ANTI-INFLAMMATORY EFFECTS OF SELECTIVE VITAMIN D RECEPTOR ACTIVATORS
Four non-inflammatory effects have been identified for selective VDRAs. Firstly, selective VDRAs regulate the plasmatic levels of PTH, with a low incidence of hypercalcaemia and hyperphosphatemia38. This is important because increased levels of phosphate and calcium may contribute to vascular calcifications6. Secondly, studies in animals showed that treatment with paricalcitol may also prevent these vascular calcifications, by interactions with vascular smooth muscle cells6. Thirdly, studies in animals showed that paricalcitol improves left ventricular function13. Finally, studies in animals also showed that paricalcitol treatment suppresses the renin-angiotensin system (RAS), which may potentially decrease the risk of hypertension, myocardial infarction and stroke11. However, further research is still needed to further investigate the last 3 effects in humans.
Recently, in the PENNY study5, a positive role of paricalcitol in conditioning endothelium-dependent vasodilatation was described. In fact, a significant endothelium-dependent vasodilatation and change in low-mediated dilation in paricalcitol treated patients was observed. Furthermore, the post-hoc analysis of PRIMO study7 showed that CKD patients randomized to the paricalcitol arm developed less atrial volume.
Chronic kidney disease is characterized by chronic inflammation39, which is strongly correlated with increased morbidity and mortality40. Such as calcitriol, selective VDRAs also have anti-inflammatory effects. In fact, an in vitro study12 demonstrated that calcitriol and paricalcitol have immunomodulatory effects, by inhibiting the maturation of dendritic cells, and consequently decreasing the production of bioactive IL-2. Furthermore, in a randomised placebo-controlled trial41 with CKD patients (stages 2 and 3), it was observed that treatment with paricalcitol for a month reduced inflammation status, by decreasing reactive C protein levels. As similar results were obtained in other studies42-44, it is possible to raise the hypothesis that paricalcitol treatment may reduce inflammation status, decreasing the risk of atherosclerosis in CKD patients.
Kidney transplantation is the best option for decreasing mortality in end-stage CKD patients. Despite this, nearly all renal transplanted patients have a decreased GFR (lower than 60 mL/min per 1.73 m2), when compared with normal native kidney function. Furthermore, vitamin D deficiency may remain in renal transplanted patients due to several reasons (e.g., corticosteroid therapy, decreased exposure to sun light). As it will be presented below, there are few studies investigating whether the benefits of paricalcitol identified for CKD patients can also be identified in kidney transplanted patients.
EFFICACY AND SAFETY OF SELECTIVE VITAMIN D RECEPTOR ACTIVATORS IN KIDNEY TRANSPLANTED PATIENTS
To our knowledge, there are only 6 published studies investigating the efficacy and safety of paricalcitol in the treatment of SHPT in kidney transplanted patients (see Table I). Four45-48 of these were reported in original articles, and 2 in conference abstracts49,50. Among thes estudies, 4 were interventional (i.e., 3 randomised, open-label, parallel two-arm studies46, 48, 50, and 1 randomised, open-label, crossover, two-arm study45), 2 were observational (1 prospective cohort study47, and 1 retrospective cohort study49), 4 included a control group45,46,48,50 and 2 did not47,49. The number of patients included varied between 1248 and 10050. The prescribed doses of oral paricalcitol were:
1 μg/day for 3 months which, if tolerated, was then increased to 2 μg/day45; 1 μg/day for 15 days which, if tolerated, was then increased to 2 μg/day48; 1 μg on alternate days47; 1 μg/day46; 1 μg 3 times a week49. Only in one of the studies50 the dose of paricalcitol was not reported. To our knowledge, there are no randomised, double blind, controlled studies about the use of paricalcitol in kidney transplanted patients.
All studies demonstrated the efficacy of paricalcitol in reducing the plasmatic levels of PTH in kidney transplanted patients45-50. The randomized, open-label, parallel two-arm study by Amer et al.48 was the largest study (100 patients) investigating the efficacy and safety of oral paricalcitol in the treatment of SHPT in kidney transplanted patients. The treatment group (51 patients) received 500 mg of elemental calcium twice a day, in combination with oral paricalcitol 1 μg/day (between days 3 and 15 post-transplant) which, in the absence of hypercalcaemia, was then increased to oral paricalcitol 2 μg/day from day 15 post-transplant onwards, until the end of study (follow-up: 1 year). The control group (49 patients) was only treated with 500 mg of elemental calcium twice a day. One year after kidney transplantation, 15 patients (29%) in the treatment group had hyperparathyroidism when compared to 31 patients (63%) in the control group (p = 0.0005). Furthermore, median plasmatic PTH concentrations identified in the treatment group (42 pg/mL) were half of those identified in the control group (85 pg/mL).
All studies also demonstrated the safety of paricalcitol in kidney transplanted patients45-50. For example, in the study by Amer et al.48 the levels of plasmatic calcium at the end of the study were significantly higher (p < 0.001) for the treat- ment group (mean = 9.9 [0.5] mg/dL) versus the control (mean = 9.7 [0.5] mg/dL). Despite this, no patients developed severe hypercalcaemia (total plasmatic calcium > 11 mg/dL with symptoms). All hypercalcaemia events were reversible by stopping calcium supplement and, in some cases, discontinuation of paricalcitol. Furthermore, in the study by Prerez et al.46 the incidence of hypercalcaemia and hyperphosphatemia was not significantly different between oral paricalcitol and no treatment. Despite the increased plasmatic calcium levels in both groups, these remained within normal ranges. Similarly, in the study by Trillini et al.45 hypercalcaemia was only observed in 2 patients taking paricalcitol 2 μg/day, and this was resolved reducing this dose to 1 μg/day. Hyperphosphatemia was never observed. Finally, in the study by Oliden et al.50, episodes of hyperphosphatemia and hypercalcaemia were not significantly different between patients taking oral paricalcitol and oral calcitriol.
Most studies also demonstrated that patients taking paricalcitol had a stable kidney function at the end of the study46-50. Only in the study by Trillini et al.45, the creatinine clearance at the end of the study was significantly lower in patients taking paricalcitol (median 60.38 [44.10–79.24 mL/min./1.73 m2) than in those in the control group (median 68.11 [52.75–87.04] mL/min./1.73 m2). However, this was probably a consequence of decreased creatinine tubular secretion, increased creatinine generation, or both, because paricalcitol is not nephrotoxic, as demonstrated by studies both in animals and in humans46-51.
Furthermore, most studies did not identify significant changes in proteinuria in patients taking paricalcitol45, 46, 48, 49. Indeed, as presented in Table I, this was only identified in the studies by Gonzalez et al.47 (mean proteinuria reduction in patients taking paricalcitol from 1.1 [0.7] g/24h to 0.7 [0.7] g/24h at month 24) and by Oliden et al.50 (patients taking paricalcitol had a significant [p = 0.02] mean pro- teinuria reduction from 481.6 [126.5]mg/L to 203 [284] mg/L).
In addition to the above, there is an ongoing study investigating whether the association between paricalcitol and dietary sodium restriction provides further proteinuria (albuminuria) reduction in non-diabetic CKD patients, on top of renin-angiotensin-aldosterone system (RAAS) blockade (ViRTUE study: vitamin D receptor activator and dietary sodium restriction to reduce residual urinary albumin excretion in chronic kidney disease)52. This study is important because albuminuria reduction is a cornerstone of CKD treatment, as albuminuria is a major contributor for CKD progression towards end-stage CKD53, and a predictor of CVD outcomes54. The first results of this study are expected starting from the 3rd quarter of 2015.
PILOT STUDIES INVESTIGATING THE TREATMENT OF VITAMIN D DEFICIENCY IN KIDNEY TRANSPLANTED PATIENTS
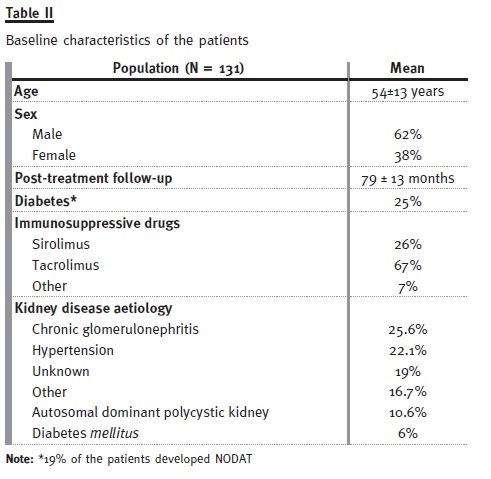
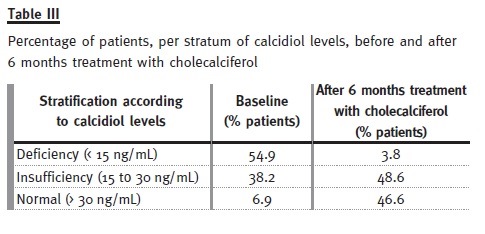
We undertook 2 prospective interventional pilot studies, investigating the efficacy and safety of the vitamin D deficiency treatment in kidney transplanted patients (see acknowledgements). In the first pilot study, kidney transplanted patients (naïve to vitamin D deficiency treatment, and on a stable dose of ACE inhibitors/ARAs) were treated with cholecal- ciferol for 6 months, according to calcidiol levels (i.e., deficiency [< 15 ng/ml]; insufficiency [15 to 30 ng/ml]; normal [> 30 ng/ml]).
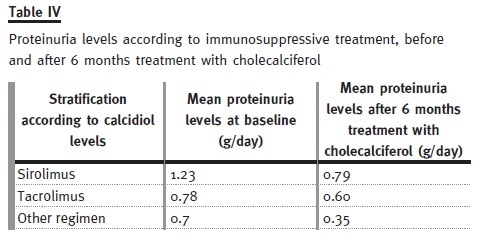
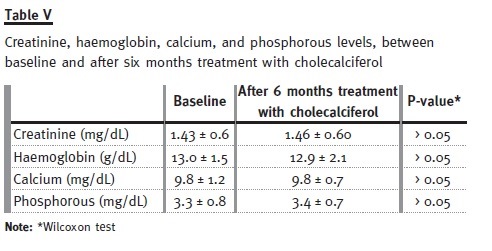
The baseline characteristics of these patients are presented in Table II. Only 6.9% of the patients had normal calcidiol levels at baseline, whereas after treatment this number increased to 46.6% (see Table III). Mean serum PTH levels decreased significantly (p = 0.005) from 139.9 ± 95 to 114 ± 94 pg/ml, and mean proteinuria levels decreased significantly (p < 0.0001) from 0.89 ± 1.2 g/day to 0.64 ± 0.9 g/day. Proteinuria was the highest in patients taking siro- limus, and it was on these patients that it reduced the most (see Table IV). Finally, no significant (p > 0.05) differences between baseline and study end were identified for plasmatic creatinine, haemoglobin, and phosphorous levels (see Table V). Therefore, our pilot study seemed to show that 6 months treatment with cholecalciferol is an effective and safe treatment for vitamin D deficiency in kidney transplanted patients.
Our second pilot study included the first 36 patients that concluded the pilot study described above. For the first 6 months, all patients (n = 36) were treated with cholecalciferol (mean dose: 2664 IU) plus calcitriol (mean dose: 0.24 ± 0.02 μg). Then, for the following 6 months, 18 out of these patients remained on the same treatment, and 17 switched to therapy with cholecalciferol (mean dose: 2664 IU) plus paricalcitol (only one patients was lost to follow-up).
The results of this second pilot study seemed to show that 6 months treatment with cholecalciferol plus calcitriol did not significantly amplify the reduction of proteinuria, in kidney transplanted patients (see Table VI). However, replacing calcitriol by paricalcitol lead to a significant intensification of the reduction in proteinuria, after 6 months of treatment (see Fig. 1).
Both pilot studies were only exploratory, as they included small sample sizes and no control groups. Despite this, these pilot studies provided valuable data to inform the larger and more robust studies that we are currently undertaking.
Three factors underline the clinical significance of undertaking such studies. Firstly, to our knowledge there are no published studies about the association of cholecalciferol plus paricalcitol. Secondly, this association successfully reduced proteinuria, in patients whose proteinuria did not reduce after 6 months of treatment with cholecalciferol plus calcitriol. Finally, the doses of paricalcitol prescribed in association with cholecalciferol were lower, than those prescribed in single therapy. This may be a strategy for reducing the costs of paricalcitol therapy.
CONCLUSION
Vitamin D deficiency has been associated with CVD, which is the leading cause of death in CKD.
Therefore, to diagnose this deficiency, and to pre- scribe an appropriate treatment, may potentially reduce morbidity and mortality in CKD patients. Vitamin D deficiency may be treated with native vitamin D, non-selective and selective VDRAs. However, selective VDRAs seem to be associated with improved survival rates, potentially due to non-inflammatory (including immunomodulatory) and anti-inflammatory effects, which were not described with other treatments.
Published studies suggest that paricalcitol is an effective and safe approach to reducing PTH levels in kidney transplanted patients. The use of calcimimetics in this population has not been approved, even though being frequently prescribed as an off label use. More research is still needed to investigate the efficacy and safety of the combination of paricalcitol plus cholecalciferol in kidney transplanted patients, but our preliminary data suggests that it may be a cost-effective approach to reduce proteinuria levels. An ongoing study will provide important evidence on the role of the association between paricalcitol and sodium restriction, on top of RAAS blockade, on proteinuria reduction.
References
1. Matias PJ, Ferreira C, Jorge C, et al. 25-Hydroxyvitamin D3, arterial calcifications and cardiovascular risk markers in haemodialysis patients. Nephrol Dial Transplant 2009;24(2):611-618. [ Links ]
2. Matias PJ, Jorge C, Ferreira C, Rodriguez M, Daurès JP, Argilés A. Cholecalciferol supple- mentation in hemodialysis patients: effects on mineral metabolism, inflammation, and cardiac dimension parameters. Clin J Am Soc Nephrol 2010;5(5):905-911. [ Links ]
3. Duranton F, Rodriguez-Ortiz ME, Duny Y, et al. Vitamin D treatment and mortality in chronic kidney disease: a systematic review and meta-analysis. Am J Nephrol 2013;37(3):239-248. [ Links ]
4. Kandula P, Dobre M, Schold JD, Schreiber MJ Jr, Mehrotra R, Navaneethan SD. Vitamin D supplementation in chronic kidney disease: a systematic review and meta-analysis of observational studies and randomized controlled trials. Clin J Am Soc Nephrol 2011 Jan;6(1):50-62. [ Links ]
5. Zoccali C, Curatola G, Panuccio V, et al. Paricalcitol and endothelial function in chronic kidney disease trial. Hypertension 2014;64(5):1005-1011. [ Links ]
6. Cardus A, Panizo S, Parisi E, Fernandez E, Valdivielso JM. Differential effects of vitamin D analogs on vascular calcification. J Bone Miner Res 2007;22(6):860-866. [ Links ]
7. Tamez H, Zoccali C, Packham D, et al. Vitamin D reduces left atrial volume in patients with left ventricular hypertrophy and chronic kidney disease. Am Heart J 2012;164(6):902- -909. [ Links ]
8. Biggar P, Kovarik J, Klauser-Braun R, et al. Paricalcitol treatment of secondary hyperpara- thyroidism in hemodialysis patients: a German-Austrian, single-arm, open-label, prospec- tive, noninterventional, observational study. Nephron Clin Pract 2014;126(1):39-50. [ Links ]
9. Martin KJ, González EA, Gellens M, Hamm LL, Abboud H, Lindberg J. 19-Nor-1-alpha-2 5-dihydroxyvitamin D2 (Paricalcitol) safely and effectively reduces the levels of intact parathyroid hormone in patients on hemodialysis. J Am Soc Nephrol 1998;9(8):1427- -1432 [ Links ]
10. Monier-Faugere MC, Mawad H, Malluche HH. Opposite effects of calcitriol and parical- citol on the parathyroid hormone-(1-84)/large carboxy-terminal-parathyroid hormone fragments ratio in patients with stage 5 chronic kidney disease. Clin J Am Soc Nephrol 2007;2(6):1255-1260. [ Links ]
11. Fryer RM, Rakestraw PA, Nakane M, et al. Differential inhibition of renin mRNA expression by paricalcitol and calcitriol in C57/BL6 mice. Nephron Physiol 2007;106(4):p76-81. [ Links ]
12. Moe SM, Zekonis M, Harezlak J, et al. A placebo-controlled trial to evaluate immuno-modulatory effects of paricalcitol. Am J Kidney Dis 2001;38(4):792-802. [ Links ]
13. McGonigle RJ, Timmis AD, Keenan J, Jewitt DE, Weston MJ, Parsons V. The influence of 1 alpha-hydroxycholecalciferol on left ventricular function in end-stage renal failure. Proc Eur Dial Transplant Assoc 1981;18:579-585. [ Links ]
14. Rech MA, Fleming JN, Moore CL. 25-hydroxyvitamin D deficiency and opportunistic viral infections after kidney transplant. Exp Clin Transplant 2014;12(2):95-100. [ Links ]
15. Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst 2006;98(7):451-459. [ Links ]
16. Wactawski-Wende J, Kotchen JM, Anderson GL, et al.with the Womens Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med 2006;354(7):684-696. [ Links ]
17. Yamshchikov AV, Desai NS, Blumberg HM, Ziegler TR, Tangpricha V. Vitamin D for treatment and prevention of infectious diseases: a systematic review of randomized controlled trials. Endocr Pract 2009;15(5):438-449. [ Links ]
18. Pitts TO, Piraino BH, Mitro R, et al. Hyperparathyroidism and 1,25-dihydroxyvitamin D deficiency in mild, moderate, and severe renal failure. J Clin Endocrinol Metab 1988;67(5):876-881. [ Links ]
19. Grotz WH, Mundinger FA, Rasenack J, et al. Bone loss after kidney transplantation: a longitudinal study in 115 graft recipients. Nephrol Dial Transplant 1995;10(11):2096-2100. [ Links ]
20. Akaberi S, Lindergård B, Simonsen O, Nyberg G. Impact of parathyroid hormone on bone density in long-term renal transplant patients with good graft function. Transplantation 2006;82(6):749-752. [ Links ]
21. Setterberg L, Sandberg J, Elinder CG, Nordenström J. Bone demineralization after renal transplantation: contribution of secondary hyperparathyroidism manifested by hypercalcaemia. Nephrol Dial Transplant 1996;11(9):1825-1828. [ Links ]
22. Anderson JL, Vanwoerkom RC, Horne BD, et al. Parathyroid hormone, vitamin D, renal dysfunction, and cardiovascular disease: dependent or independent risk factors? Am Heart J 2011;162(2):331-339. [ Links ]
23. Zittermann A, Kuhn J, Dreier J, Knabbe C, Gummert JF, Börgermann J.. Vitamin D status and the risk of major adverse cardiac and cerebrovascular events in cardiac surgery. Eur Heart J 2013;34(18):1358-1364. [ Links ]
24. Goldsmith DJ, Cunningham J. Mineral metabolism and vitamin D in chronic kidney disease–more questions than answers. Nat Rev Nephrol 2011;7(6):341-346. [ Links ]
25. Courbebaisse M, Xu-Dubois YC, Thervet E, et al. Cholecalciferol supplementation does not protect against renal allograft structural and functional deterioration: a retrospec- tive study. Transplantation 2011;91(2):207-212. [ Links ]
26. Lee JR, Dadhania D, August P, Lee JB, Suthanthiran M, Muthukumar T.. Circulating levels of 25-hydroxyvitamin D and acute cellular rejection in kidney allograft recipients. Transplantation 2014;98(3):292-299. [ Links ]
27. Holick MF. Vitamin D deficiency. N Engl J Med 2007;357(3):266-281. [ Links ]
28. Lewis R. Mineral and bone disorders in chronic kidney disease: new insights into mechanism and management. Ann Clin Biochem 2012;49(Pt 5):432-440. [ Links ]
29. Shvil Y, Naveh-Many T, Barach P, Silver J. Regulation of parathyroid cell gene expression in experimental uremia. J Am Soc Nephrol 1990;1(1):99-104. [ Links ]
30. Cozzolino M, Ketteler M, Martin KJ, Sharma A, Goldsmith D, Khan S.. Paricalcitol- or cinacalcet-centred therapy affects markers of bone mineral disease in patients with secondary hyperparathyroidism receiving haemodialysis: results of the IMPACT-SHPT study. Nephrol Dial Transplant 2014;29(4):899-905. [ Links ]
31. Cozzolino M, Brancaccio D, Cannella G, et al. with the FARO Study Group. VDRA therapy is associated with improved survival in dialysis patients with serum intact PTH ≤ 150 pg/mL: results of the Italian FARO Survey. Nephrol Dial Transplant 2012;27(9):3588-3594. [ Links ]
32. Wolf M, Shah A, Gutierrez O, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int 2007;72(8):1004-1013. [ Links ]
33. Tentori F, Hunt WC, Stidley CA, et al. Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int 2006;70(10):1858-1865. [ Links ]
34. Kalantar-Zadeh K, Kuwae N, Regidor DL, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 2006;70(4):771-780. [ Links ]
35. Teng M, Wolf M, Ofsthun MN, et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol 2005;16(4):1115-1125. [ Links ]
36. Shoji T, Shinohara K, Kimoto E, et al. Lower risk for cardiovascular mortality in oral 1alpha-hydroxy vitamin D3 users in a haemodialysis population. Nephrol Dial Transplant 2004;19(1):179-184. [ Links ]
37. Teng M, Wolf M, Lowrie E, et al. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med 2003;349(5):446-456. [ Links ]
38. Ketteler M, Martin KJ, Wolf M, et al. Paricalcitol versus cinacalcet plus low-dose vitamin D therapy for the treatment of secondary hyperparathyroidism in patients receiving haemodialysis: results of the IMPACT SHPT study. Nephrol Dial Transplant 2012;27(8): 3270-3278.
39. Lo WK. Serum parameters, inflammation, renal function and patient outcome. Contrib Nephrol 2006;150:152-155. [ Links ]
40. Tentori F, Blayney MJ, Albert JM, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2008;52(3):519-530. [ Links ]
41. Alborzi P, Patel NA, Peterson C, et al. Paricalcitol reduces albuminuria and inflammation in chronic kidney disease: a randomized double-blind pilot trial. Hypertension 2008;52(2):249-255. [ Links ]
42. Sochorová K, Budinsky V, Rozková D, et al. Paricalcitol (19-nor-1,25-dihydroxyvitamin D2) and calcitriol (1,25-dihydroxyvitamin D3) exert potent immunomodulatory effects on den- dritic cells and inhibit induction of antigen-specific T cells. Clin Immunol 2009;133(1):69-77. [ Links ]
43. Izquierdo MJ, Cavia M, Muñiz P, et al. Paricalcitol reduces oxidative stress and inflammation in hemodialysis patients. BMC Nephrol 2012;13:159. [ Links ]
44. Navarro-Gonzalez JF, Donate-Correa J, Mendez ML, Fuentes MM, García-Pérez J, Mora-Fernández C. Anti-inflammatory profile of paricalcitol in hemodialysis patients: a prospective, open-label, pilot study. J Clin Pharmacol 2013;53(4):421-426. [ Links ]
45. Trillini M, Cortinovis M, Ruggenenti P, et al. Paricalcitol for secondary hyperparathyroidism in renal transplantation. J Am Soc Nephrol 2015;26(5):1205-1214. [ Links ]
46. Perez V, Sanchez-Escuredo A, Lauzurica R, et al. Magnetic bead-based proteomic technology to study paricalcitol effect in kidney transplant recipients. Eur J Pharmacol 2013;709(1-3):72-79. [ Links ]
47. Gonzalez E, Rojas-Rivera J, Polanco N, et al. Effects of oral paricalcitol on secondary hyperparathyroidism and proteinuria of kidney transplant patients. Transplantation 2013;95(7):e49-52. [ Links ]
48. Amer H, Griffin MD, Stegall MD, et al. Oral paricalcitol reduces the prevalence of post- transplant hyperparathyroidism: results of an open label randomized trial. Am J Transplant 2013;13(6):1576-1585. [ Links ]
49. Valga EF, De la Flor JC, Calvo M, et al. Effects of paricalcitol in calcium-phosphorus metabolism, renal function and roteinuria in kidney transplantation: 1594. Transplantation 2012;94(10S):889. [ Links ]
50. Oliden C, Leon L, Tavera M, et al. Oral paricalcitol versus oral calcitriol for the treatment of secondary hyperparathyroidism in renal transplantation: 1707. Transplantation 2012;94(10S):898. [ Links ]
51. Coyne DW, Andress DL, Amdahl MJ, Ritz E, de Zeeuw D. Effects of paricalcitol on calcium and phosphate metabolism and markers of bone health in patients with diabetic nephropathy: results of the VITAL study. Nephrol Dial Transplant 2013;28(9):2260-2268. [ Links ]
52. Keyzer CA, de Jong MA, Fenna van Breda G, et al. with the Holland Nephrology Study (HONEST) Network. Vitamin D receptor activator and dietary sodium restriction to reduce residual urinary albumin excretion in chronic kidney disease (ViRTUE study): rationale and study protocol. Nephrol Dial Transplant 2015 Mar 4. [ Links ]
53. Jafar TH, Stark PC, Schmid CH, et al. with the AIPRD Study Group. Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 2001;60(3):1131-1140. [ Links ]
54. Hemmelgarn BR, Manns BJ, Lloyd A, et al. wit the Alberta Kidney Disease Network. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303(5):423-429.
Prof. Doutor Aníbal Ferreira
Nova Medical School – Faculdade de Ciências Médicas Universidade Nova de Lisboa
Serviço de Nefrologia,
Hospital Curry Cabral Centro Hospitalar de Lisboa Central
Rua da Beneficência 8, 1069-166 Lisboa
E-mail: anibalferreira@netcabo.pt
ACKNOWLEDGEMENTS
The pilot studies described in this literature
Conflict of Interest statement: A.F. reported having received research grants from Abbvie, Amgen, Genzyme, and Shire, conference sponsorships from Abbvie, Amgen, Fresenius Medical Care, Genzyme, and Shire, and to have participated in advisory boards promoted by Abbvie, Amgen, Fresenius Medical Care, Genzyme, and Shire. A.G.C. and D.M. have participated in an advisory board promoted by Abbvie. P.L.N. has participated in advisory boards promoted by Abbvie, Genzyme, and Amgen, and received research grants from Abbvie, Genzyme, and Amgen. F.M. participated in advisory boards promoted by Novartis, Astellas, Abbvie, and OmPharma. J.B.P. works at a CRO, which received scientific consulting fees from Abbvie. No other conflicts of interest were declared.
Received for publication: 14/07/2015
Accepted in revised form: 28/08/2015














