Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Portuguese Journal of Nephrology & Hypertension
Print version ISSN 0872-0169
Port J Nephrol Hypert vol.29 no.2 Lisboa June 2015
CASE REPORT
Pleuroperitoneal communication on peritoneal dialysis patients – presentation of four cases
Comunicação pleuroperitoneal em diálise peritoneal – quatro casos clínicos
Ana Rita Martins1, Patrícia Branco1, Margarida Gonçalves1, Marta Marques3, Sophia P Afonso2, Augusta Gaspar1, José Diogo Barata1
1Nephrology Department, Hospital Santa Cruz. Carnaxide, Portugal
2 Nuclear Medicine Department, Hospital Santa Cruz. Carnaxide, Portugal
3Cardiothoracic Surgery Department, Hospital Santa Cruz. Carnaxide, Portugal
ABSTRACT
Peritoneal dialysis is an established treatment option for patients with end-stage renal disease. As this procedure causes high pressure in the abdomen, it might lead to peritoneopleural leakage (hydrothorax). The latter is an uncommon non-infectious complication of peritoneal dialysis patients, possibly secondary to trans-diaphragmatic fluid leakage through a pleuroperitoneal communication. The aetiology of this condition is still unclear. A variety of mechanisms have been proposed: congenital diaphragmatic defects; pleuroperitoneal pressure gradient; lymph drainage disorder; acquired diaphragmatic defects that are a consequence of increased intra-abdominal pressure during the dwell period of peritoneal dialysis, such as fenestrations or acquired scarcity of muscle fibres in the tendinous part of the diaphragm. Peritoneal scintigraphy (99mTc-MAA peritoneoscintigraphy) is a useful diagnostic tool in the management of suspected pleuroperitoneal communication. We report four cases of hydrothorax related to pleuroperitoneal communication in 290 patients treated with chronic peritoneal dialysis, between 1999 and 2014, at our PD Unit. Here we analyse our departments incidence of this complication and also present own experience in diagnosing and treating it.
Key-Words: Continuous ambulatory peritoneal dialysis; peritoneopleural communication; pleurodesis; secondary hydrothorax; technetium-99m-macroaggregated albumin scintigraphy (99mTc-MAA).
RESUMO
A diálise peritoneal é uma opção válida para o tratamento da doença renal crónica estádio 5. Como este procedimento provoca um aumento da pressão intra-abdominal, pode causar uma fuga pleuroperitoneal (hidrotórax). Esta complicação não infecciosa, sendo rara, pode ser fatal para doentes em diálise peritoneal; provavelmente secundária à passagem trans-diafragmática de líquido através de uma comunicação pleuroperitoneal. A sua etiologia não está ainda definida, foram propostas diversas causas: defeitos congénitos do diafragma; gradiente de pressão pleuroperitoneal; distúrbios da drenagem linfática; defeitos adquiridos do diafragma, como o aparecimento de fenestrações ou a escassez de fibras musculares diafragmáticas, situações agravadas pela pressão intra-abdominal durante a permanência de dialisante na diálise peritoneal. A cintigrafia peritoneal (peritoneocintigrafia 99mTc-MAA) é uma ferramenta útil no diagnóstico de comunicação pleuroperitoneal. Apresentamos quatro casos de hidrotórax associados a comunicação pleuroperitoneal num total de 290 doentes tratados com diálise peritoneal entre 1999 e 2014, na nossa Unidade. Analisamos a incidência, estratégia diagnóstica e terapêutica desta complicação.
Palavras-Chave: Cintigrafia com macroagregados de albumina marcados com tecnécio-99m; comunicação pleuroperitoneal; diálise peritoneal continua e ambulatória; hidrotórax secundário; pleurodese.
INTRODUCTION
Peritoneal dialysis (PD) is an effective mode of management for end-stage renal disease (ESRD). The main complications of PD are either infectious (such as peritonitis and exit site infections) or pressurerelated (such as abdominal wall hernias and gastrooesophageal reflux). Acute hydrothorax is a rare complication in PD patients that can lead to PD suspension and consequent switch to haemodialysis (HD).
As described in 1967 by Edward and Unger, hydrothorax corresponds to the presence of PD fluid in the pleural cavity due to movement of dialysate under increased intra-abdominal pressure from peritoneal to pleural cavity, through congenital or acquired defects in the diaphragm1. The mean incidence of hydrothorax is highly variable in the bibliography, with estimates ranging from 1.6% to 10%2-5.
Clinical presentation may sometimes be asymptomatic, but, in some patients, it may cause dyspnoea, a sudden diminution in dialysis adequacy and/or poor ultrafiltration rate, that does not improve with hypertonic peritoneal exchanges.
According to the bibliography, right-sided pleural effusion is more common.2,4-6 Its aetiopathogenesis is not fully understood. In a recent review, Guest proposed that right-sided PD-related hydrothorax pathophysiology results from a mechanism which comprises: congenital or acquired porous diaphragm more common in the right hemidiaphragm; intestinal circulation sweeps fluid preferentially to the abdominal right upper quadrant; highest hydrostatic pressure in the pelvis and lowest in the suprahepatic region during inspiration, due to the outward movement of the ribs which enlarges the space in the right upper quadrant and a piston-like motion of liver capsule shunts fluid across the diaphragmatic vent6.
Thoracentesis should be performed for diagnosis and relief of symptoms. If the pleural fluid sample shows that the glucose concentration is higher than plasmas, a positive diagnosis can be supposed, but 99mTc-MAA peritoneoscintigraphy has been a useful diagnostic tool in our experience2.
Treatment includes:
1. Thoracentesis – stop PD and engage in temporary HD or PD with lower intraperitoneal volumes (the dialysate in the pleural cavity may function as a sclerosing agent);
2. Pleurodesis with talc, tetracycline, bleomycin, autologous blood or with collagen fleece;
3. Thoracoscopic (by video or direct surgery) for repairing and patching the diaphragmatic defects identified.
CASE REPORTS
Between January 1999 and December 2012, four patients undergoing PD in our Peritoneal Dialysis Department (one man and three women; mean age 55 years) developed acute hydrothorax due to pleuroperitoneal communication (prevalence rate: 1.37%).Hydrothorax was diagnosed after 2, 6, 15 and 11 months. Table I illustrates the summary of the cases.
Case 1
Our first case was a 61-year-old woman with chronic kidney disease (CKD), due to congenital bilateral ureterohydronephrosis, receiving PD since January 2012. Her 24 hours diuresis was about 2300 cc.
After two months on CAPD, the patient attended the emergency room with dyspnoea, peripheral oedema, hypertension and low ultrafiltration. Her vital signs were normal, but she had decreased breath sounds over the lower half of the right lung. She was otherwise well and afebrile. Her ultrafiltration with hypertonic solutions was only 200 cc per cycle.
Chest radiography confirmed a large pleural effusion in the right hemithorax. Her ECG was unchanged. Her serum biochemistry and haematology were unchanged and the inflammatory markers were not raised (Figs.1 and 2). A chest tube was placed and the pleural fluid analysis showed clear yellow fluid with 67 nucleated cells, glucose 212 mg/dL (blood glucose 104 mg/dL), protein 0.2 g/dL and lactate dehydrogenase (LDH) 63 U/L (blood LDH 560 U/L). Cultures of pleural fluid were negative. As the fluid was a transudate (Table I), an intraperitoneal injection of 99mTc-MAA was performed via the Tenckhoff catheter and the scintigraphy images revealed positive uptake of the chest and upper abdomen by diffuse increase in radioactivity in the right pleural and peritoneal cavities (Fig. 3).
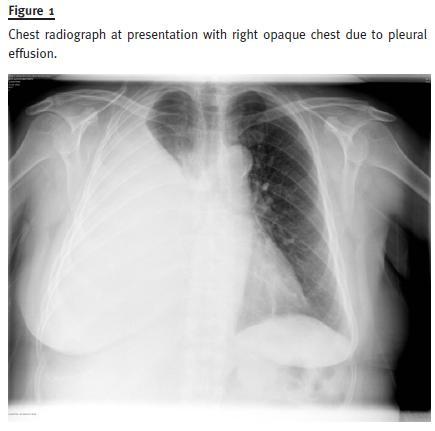
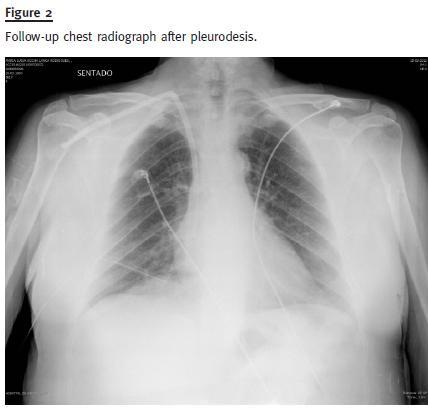
The patient remained on HD by a tunnelled femoral catheter for about three months. Pleurodesis with 100cc of talc instillation and water was performed to close the communication; the patient recovered well and returned to PD. She had no other related complications in the follow-up.
Case 2
A 56-year-old woman with CKD of unknown origin under haemodialysis treatment since February 2002, who had been transferred to peritoneal dialysis, five years later, due to vascular access exhaustion. She was anuric.
Six months after starting PD and following an upper respiratory infection, the patient developed dyspnoea and fatigue, which worsened progressively. Ultrafiltration with hypertonic solutions was only 350 cc per cycle. Physical findings included an arterial oxygen saturation of 91%, raised jugular venous pressure, right hemithorax was stony dull on percussion and breath sounds were very much reduced. The chest radiography showed a right pleural effusion. Her serum biochemistry and haematology were unchanged. Inflammatory markers were not raised.
Thoracentesis revealed a transudate process (Table I) – clear yellow fluid with 37 nucleated cells, glucose 173 mg/dL (blood glucose 101 mg/dL), protein 0.3 g/dL and LDH 45 U/L (blood LDH 483 U/L). Cultures of pleural fluid were sterile. As the fluid was a transudate (Table I), an intraperitoneal injection of 99mTc-MAA was administered via the Tenckhoff catheter.
According to the clinical, radiological and cytological findings, the diagnosis of the peritoneopleural communication was highly probable.
In accordance with patient preference, PD was discontinued and she was switched to HD.
Case 3:
We report also a 48-year-old Caucasian male with CKD of unknown aetiology treated with CAPD since January 2000. His diuresis was around 500 cc per day. Fifteen months after beginning CAPD he wasadmitted to our hospital reporting orthopnea, shortly after having received intraperitoneal vancomycin for Staphylococcus aureus peritonitis. The patient had also reported a lower ultrafiltration in recent days.
Physical examination revealed decreased breath sounds on the right lung and a chest X-ray showed ipsilateral pleural effusion. Examination of the cardiovascular system was unremarkable. Ultrafiltration with hypertonic solutions was around 400 cc per cycle.
Pleural fluid analysis revealed a clear yellow fluid with 29 nucleated cells, glucose 162 mg/dL (blood glucose 105 mg/dL), protein 0.6 g/dL and LDH 52 U/L (blood LDH 576 U/L). Cultures of pleural fluid were sterile.
Given the suspicion of pleuroperitoneal communication, an isotopic peritoneal scintigraphy with 99mTc-MAA was performed. The images of the chest and upper abdomen revealed diffused increase in radioactivity in the right hemithorax. Intraperitoneal volumes were reduced and the patient was submitted to thoracic drainage, pleurodesis with talc and direct surgery to correct malformations.
The patient remained on HD for only one month, and after that period he returned to PD, with no further complication in the following years.
Case 4:
Our last case reports a 56-year-old Black woman with CKD secondary to hypertensive nefroangiosclerosis, on CAPD for 11 months. Her diuresis was about 700 cc per day.
After eleven months on CAPD the patient had presented progressive shortness of breath, fatigue with minimal effort and sub-febrile temperature for about four days before admission. She was observed in consultation and was diagnosed with a respiratory infection and medicated with azithromycin. As there was no clinical improvement, she showed up at our emergency department. Physical examination revealed decreased breath sounds on the right lung and a chest X-ray showed a right pleural effusion. Abdominal examination showed tender hepatomegaly. Her inflammatory markers were negative. Ultrafiltration with hypertonic solutions was only 200 cc per cycle.
A peritonitis to Staphylococcus epidermidis was treated with intraperitoneal vancomycin until two weeks earlier.
A chest tube was placed and the patient was submitted to pleurodesis. Pleural fluid analysis revealed a clear yellow fluid with 70 nucleated cells, glucose 149 mg/dL (blood glucose 110 mg/dL), protein 0.4 g/dL and LDH 78 U/L (blood LDH 487 U/L). The cultural tests (blood and pleural fluid) were negative and two litres of pleural fluid were drained without further relapse of the pleural effusion.
Respecting the patient´s option, pleural communication was not treated. She was, therefore, transferred to HD.
DISCUSSION
Peritoneal dialysis is a well-established renal replacement therapy. The main complications of PD are infectious (peritonitis and exit site infections) or pressurerelated (abdominal wall hernias).7 The infusion of liquid into the peritoneal cavity in PD leads to an increase in intra-abdominal pressure and might cause peritoneal leakage (hydrothorax), most commonly on the right side8. Although this is not a common complication, it is associated with high drop-out rates.
In our PD Unit, we have observed a peritoneopleural communication incidence of 1.37%, lower than in other series. According to the literature most of the cases were female and the same pattern emerged in our cases. Polycystic kidney disease is the most frequent cause for CKD associated with this complication, due to a higher intra-abdominal pressure6 and to a generalized connective tissue weakness that may contribute to inherent diaphragmatic weakness9. Nevertheless, none of our four patients had polycystic kidney disease.
In the current literature it is not clear an association between the incidence of pleuroperitoneal communication and the mode of PD (CAPD or APD).
However, Lam et al. reported, in a series of 23 cases of retroperitoneal leakage, a higher incidence among patients on CAPD (22 vs. 1)10. The same trend was observed in our series (4 vs 0).
Isolated right-sided hydrothorax is common in pleuroperitoneal communication of peritoneal dialysis, as was the case in our four patients. Gagnon and Daniels11 proposed the existence of embryonic remnant, which allows fluids to pass from the peritoneal cavity to the right pleural space. Other authors defend that the higher prevalence of right-side cases is due to diaphragmatic left protection to the heart6.
According to a series with 47 reported cases, pleural effusions were right-sided in 87%12. Bilateral pleuroperitoneal communication is uncommon and should be related to malformation.
The mechanism of pleural fluid accumulation remains unclear. Most investigators agree with two major mechanisms in the formation of pleural effusion in PD: lymphatic drainage disorders and diaphragmatic defect. The latter occurs by congenital or acquired diaphragmatic defects, due to an increase in intra-abdominal pressure when instilling peritoneal dialysis fluid6. Localized absence of diaphragmatic muscle fibers13,14 has been demonstrated by scintigraphy and autopsy specimens.
Several researchers suggest that the rate of transdiaphragmatic passage of the radionuclide can fall under two mechanisms: on the one hand, if the passage occurs in few minutes it is more likely to be a diaphragmatic defect; on the other hand, a lymphatic drainage is highly suspected when the liquid takes longer to move7.
In three of our cases (2 to 4) the authors have identified a predisposing factor (upper respiratory infection and/or acute peritonitis) prior to the pleuroperitoneal communication diagnosis. In our first case, the earlier occurrence after the onset of CAPD suggests a congenital aetiology. Chow et al. proposed that patients with previous peritonitis presented higher risk of developing hydrothorax, probably as a result of the weakening of diaphragmatic tissue during infection15.
The onset of this complication can vary from days to years after starting PD. About half of the cases occur within one month of starting PD, which probably result from congenital diaphragmatic defects. The remaining cases are probably the result of acquired diaphragmatic defects6. Our four cases occurred between 2 and 15 months after the initiation of PD.
Thus, our first case suggests a congenital defect, as it has happened just two months after initiating PD.
The diagnosis of pleuroperitoneal communication in PD must be based on clinical symptoms, such as dyspnoea, reduced ultrafiltration and pleural effusion in the chest X-ray. The differential diagnosis of pleural effusion in dialysis patients is extensive. Therefore, other causes of dyspnoea should be ruled out, such as salt retention, heart failure, hypoproteinaemia, infections or neoplasia.
When pleural fluid collected from thoracentesis shows a higher glucose concentration compared to plasmas, a diagnosis of pleuroperitoneal communication can be supposed – a sweet hydrothorax.
Some authors consider that glucose pleural effusion/serum ratio > 1 is consistent with pleuroperitoneal communication and may dispense performing scintigraphy for diagnosis5,15. However, the peritoneal scintigraphy was useful in our cases. In all of them, a glucose pleural effusion/serum ratio was superior to 1, but in case 4 the ratio was only slightly above the cut off (1.35), therefore, the scintigraphy was useful to confirm this condition. Furthermore, there are some cases described where an atypical biochemistry of the pleural fluid was observed. This usually happened when the fluid has been in the pleural cavity for a prolonged period and lymphatics have partially reabsorbed it6.
Peritoneal scintigraphy is a useful diagnostic tool in the management of this PD complication6. The presence of radioactivity in the pleural space and in the peritoneal cavity, after injection of non-absorbable 99mTc-MAA along the dialysis solution via the Tenckhoff catheter, reflects hydrothorax secondary to a communication between the two cavities7. We have selected 99mTc-MAA from several available radiopharmaceuticals.
In our cases, none of the patients underwent magnetic resonance (MR) peritoneography. Prokesch et al. evaluated the diagnostic value of MR peritoneography in complications of PD. This procedure was performed with the peritoneal cavity filled and after complete drainage of the contrast material-dialysate mixture. According to Prokesch et al., in patients with right-sided leaks, MR peritoneography with multiplane imaging was useful to evaluate all parts of the diaphragm.
Coronal and sagittal images may be obtained for assessment of the lateral and posterior recesses16.
Despite some evidence in favour of MR (well tolerated by the patients, iodinated contrast material is not used and excellent tissue contrast), the majority of pleuroperitoneal cases do not require special imaging to confirm the diagnosis. Furthermore MR is not always available, especially in an emergency context.
In a recent publication, the authors performed in addition to a peritoneal scintigraphy with Tc-99m macro-aggregated albumin, a single-photon-emission computed tomography and a computed tomography (SPECT/CT) to identify the leakage site17.
In terms of management, emergent large volume thoracocentesis is occasionally required. Temporary HD may be required, especially for those with minimal residual renal function. For patients who maintain significant diuresis, PD may be continued at low volumes with dry night. It is recommended to wait around two weeks before an attempt to switch back to standard PD settings1. If the conservative approach fails, the treatment methods reported in the bibliography include pleurodesis or surgery11. Chemical pleurodesis is performed with tetracycline, talc, fibrin, glue, autoblood, Nocardia rubracell wall skeleton (N-CWS) and OK-432. An insoluble substance, such as talc, will remain in the pleural cavity without systemic absorption, thus proportionating permanent pleurodesis18,19. There is no evidence that one method is better than the other. As pleuroperitoneal communication is a clinical situation with little relevance outside the context of PD, conservative treatment may be the most suitable option for patients who desire to be transferred to HD1.
Video-assisted thoracoscopic surgery allows malformations diaphragmatic correction by direct visualization.
If the condition is associated with a morphological disorder, direct repair by thoracotomy is mandatory. Although surgery is associated with a high rate of success, it must be reserved as last treatment option because it is an invasive approach6,16,20.
In our experience, talc pleurodesis was a safe and reliable treatment option that allowed sustained maintenance of PD with no recurrence in two cases.
The other two patients opted to be transferred to HD. In those former cases, the decision was to engage in peritoneal rest with temporary transition to HD.
Pleurodesis was the treatment option in two cases and one of them was also treated by surgery.
CONCLUSION
Transudate pleural effusion due to pleuroperitoneal communication is an uncommon but serious complication in PD patients. It can lead to acute respiratory failure and is an important cause of PD dropout.
Radionuclide peritoneal scintigraphy is a simple, safe, rapid and effective tool to identify peritoneopleural communication.
Many therapeutic strategies for this complication are employed, ranging from conservative methods like reduction on dialysate volume and the transitory interruption of PD, to more invasive therapies as the closure of diaphragmatic defects by videothoracoscopy with or without pleurodesis.
The fact that two of four patients remained in PD without recurrence of the pleural effusion was a reasonable outcome. Pleurodesis can therefore be recommended in well-informed patients, who have a strong motivation to pursue PD. We can also conclude that pleuroperitoneal connections associated with PD do not necessarily lead to cessation of PD and conversion to HD.
References
1. Edwards SR, Unger AM. Acute hydrothorax – a new complication of peritoneal dialysis. JAMA 1967;199(11):853-855. [ Links ]
2. Díaz Mancebo R, del Peso Gilsanz G, Rodriguez M, et al. Pleuroperitoneal communication in patients on peritoneal dialysis. One hospital´s experience and review of the literature. Nefrologia 2011;31(2):213-217. [ Links ]
3. Nomoto Y, Suga T, Nakajima K, et al. Acute hydrothorax in continuous ambulatory peritoneal dialysis – a collaborative study of 161 centers. Am J Nephrol 1989;9(5):363-367. [ Links ]
4. Michel C, Devy A, Lavaud F, Lavaud S, Lebargy F. [A sweet hydrothorax] Presse Med 2001;30(28):1401-1403. [ Links ]
5. Momenin N, Colletti PM, Kaptein EM. Low pleural fluid-to-serum glucose gradient indicates pleuroperitoneal communication in peritoneal dialysis patients: presentation of two cases and a review of the literature. Nephrol Dial Transplant 2012;27(3):1212-1219. [ Links ]
6. Guest S. The curious right-sided predominance of peritoneal dialysis-related hydrothorax. Clin Kidney J 2015;8(2):212-214. [ Links ]
7. Kennedy C, McCarthy C, Alken S. et al. Pleuroperitoneal leak complicating peritoneal dialysis: A case series. Int J Nephrol 2011;526753. doi: 10.4061/2011/526753. [ Links ]
8. Yao-Nan Y, Yu-Ming F, Chuang-Hsin C, et al. Diagnosis of peritoneopleural communication with 99mTc-MAA. Scintigraphy in a patient with continuous ambulatory peritoneal dialysis: A case report and literature review. Ann Nucl Med Sci 2004;17:51-55. [ Links ]
9. Fletcher S, Turney JH, Brownjohn AM. Increased incidence of hydrothorax complicating peritoneal dialysis in patients with adult polycystic kidney disease. Nephrol Dial Transplant 1994;9(7):832-833. [ Links ]
10. Lam MF, Lo WK, Tse KC, et al. Retroperitoneal leakage as a cause of acute ultrafiltration failure: its associated risk factors in peritoneal dialysis. Perit Dial Int 2009;29(5):542-547. [ Links ]
11. Mahale AS, Katyal A, Khanna R. Complications of peritoneal dialysis related to increase intra-abdominal pressure. Adv Perit Dial 2003;19:130-135. [ Links ]
12. Garcia-Maldonado B, Guerrero-Ortiz M, Gómez-Fuentes JR, et al. Pleuroperitoneal communication in peritoneal dialysis patient. Nefrologia 2012;32(4):551-552. [ Links ]
13. Tokmak H, Mudun A, Türken C, Sanli Y, Cantez S, Bozfakiouglu S. The role of peritoneal scintigraphy in the detection of continuous ambulatory peritoneal dialysis complications. Ren Fail 2006;28(8):709-713. [ Links ]
14. García Ramón R, Carrasco AM. Hydrothorax in peritoneal dialysis. Perit Dial Int 1998;18(1)5-10. [ Links ]
15. Chow CC, Sung YJ, Cheung CK, Hamilton-Wood C, Lai KN. Massive hydrothorax in continuous ambulatory peritoneal dialysis: diagnosis, management and review of the literature.N Z Med J 1988;101(850):475-477. [ Links ]
16. Prokesch RW, Schima W, Schober E, Vychytil A, Fabrizii V, Bader TR. Complications of continuous ambulatory peritoneal dialysis: findings on MR peritoneography. Am J Roentgenol 2000;174(4)987-991. [ Links ]
17. Nishiyama K, Fukushima K, Jo T, et al. SPECT/CT to diagnose pleuroperitoneal communication-associated hydrothorax in peritoneal dialysis. Kidney Int 2015;87(4):866. [ Links ]
18. Nishina M, Iwazaki M, Koizumi M, et al. Case of peritoneal dialysis-related acute hydrothorax, which was successfully treated by thoracoscopic surgery, using collagen fleece. Tokai J Exp Clin Med 2011;36(4):91-94. [ Links ]
19. Mak SK, Chan MW, Tai YP, et al. Thoracoscopic pleurodesis for massive hydrothorax complicating CAPD. Perit Dial Int 1996;16(4):421-423. [ Links ]
20. Allen SM, Matthews HR. Surgical treatment of massive hydrothorax complicating continuous ambulatory peritoneal dialysis. Clin Nephrol 1991;36(6):299-301. [ Links ]
21. Rajnish A, Ahmad M, Kumar P. Peritoneal scintigraphy in the diagnosis of complications associated with continuous ambulatory peritoneal dialysis. Clin Nucl Med 2003;28(1):70-71. [ Links ]
22. Lemos S, Sá HO, Carmo C, et al. Hidrotórax – complicação rara em diálise peritoneal crónica ambulatória: a propósito de dois casos. Rev Port Nefrol Hipert 2004;18(4):235-241. [ Links ]
Conflict of interest statement: None declared
Drª Ana Rita Mateus Martins
Department of Nephrology, Hospital Santa Cruz, Centro Hospitalar de Lisboa Ocidental.
Avenida Professor Reinaldo dos Santos, 2790-240 Carnaxide, Oeiras, Portugal.
E-mail: anarita.mateus@gmail.com
Received for publication: 19/02/2015
Accepted in revised form: 20/05/2015














