Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.29 no.2 Lisboa jun. 2015
CASE REPORT
Posterior reversible encephalopathy syndrome as a kidney transplant immunosuppressive therapy side effect
Síndrome da leucoencefalopatia posterior reversível como efeito colateral da terapêutica imunossupressora no transplante renal
Maria João Melo, Célia Nascimento, José Guerra, João Gonçalves, Alice Santana
Nephrology and Renal Transplantation Department, Hospital de Santa Maria, Centro Hospitalar Lisboa Norte, EPE, Portugal
ABSTRACT
Successful kidney transplant improves the quality of life and reduces the mortality risk for most patients when compared to maintenance dialysis. We present the case of a 61-year-old woman with chronic kidney disease stage 5-d who received a deceased donor renal transplant. At 21 months after transplantation she developed early post-transplant diabetes and was diagnosed with Pneumocystis jirovecii pneumonia. Her basal creatinine level was 1.1 mg/dL. The patient was admitted with a 7-day history of fever, productive cough, anorexia and right hand altered mobility and sensibility. She was diagnosed with Aspergillus fumigatus pneumonia and the encephalocranial magnetic resonance was suggestive of posterior reversible encephalopathy syndrome, possibly related to high tacrolimus serum levels. Approximately a month later, a renal ultrasound scan and further abdomen computed tomography scan (CT) suggested a tumour and the patient was submitted to right nephrectomy. After discharge, she has been regularly evaluated, maintaining a stable serum creatinine of 2.3 mg/dL, no symptoms associated. This clinical case highlights the myriad of potential complications that transplant recipients are vulnerable to when receiving immunosuppressive therapy.
Key-Words: Immunosuppressants; kidney transplant; posterior reversible encephalopathy syndrome.
RESUMO
O transplante renal bem sucedido melhora a qualidade de vida e reduz o risco de mortalidade para a maioria dos doentes quando comparado à terapêutica dialítica de manutenção. Os autores apresentam o caso clinico de uma doente de 61 anos com doença renal crónica estadio 5-d que foi transplantada com um rim de dador cadaver. A doente desenvolveu diabetes no periodo pós-transplante precoce e teve uma pneumonia a Pneumocystis jirovecii. A creatinina basal era de cerca de 1,1 mg/dL. A doente foi admitida por quadro com cerca de 7 dias de febre, tosse produtiva, anorexia e mobilidade e ensibilidade alteradas na mão direita. Foi-lhe diagnosticada pneumonia a Aspergillus fumigatus e a ressonância magnética craneoencefálica foi sugestiva de Síndrome de Leucoencefalopatia Posterior Reversível, possivelmente relacionada com níveis séricos elevados de tacrolimus. Cerca de um mês depois, aquando da realização de ecografia renal e posteriormente tumografia computorizada, foi-lhe diagnosticada neoplasia renal, tendo sido submetida a nefrectomia direita. Após a alta a doente tem sido avaliada em consulta, mantendo creatinina sérica estável de cerca de 2,3 mg/dL, sem sintomatologia associada. Este caso clinico evidencia a multiplicidade de potenciais complicações a que os doentes transplantados renais estão sujeitos em virtude da terapêutica imunosupressora a que são submetidos.
Palavras-Chave: Síndrome de leucoencefalopatia posterior reversível; terapêutica imunosupressora; transplante renal.
INTRODUCTION
Successful kidney transplant improves the quality of life and reduces the mortality risk for most patients when compared to maintenance dialysis. However, patients require close follow-up after transplantation since they are on complex immunosuppressive regimens that render them susceptible to infection, malignancy, cardiovascular disease, and other complications.
In addition, patients often have multiple comorbidities due to, or as a cause of, their underlying renal disease1-4.
The authors present this clinical case as an example of the large spectrum of pathology that can affect kidney transplant recipients.
CASE REPORT
We present the case of a 61-year-old woman with chronic kidney disease (CKD) stage 5-d secondary to probable chronic glomerulonephritis (kidney biopsy was inconclusive) who received a deceased donor kidney allograft 24 months before this admission.
The induction immunosuppressive protocol was basiliximab, tacrolimus, prednisone and mycophenolate mofetil, and maintenance immunosuppression was tacrolimus prednisone and mycophenolate mofetil.
After transplantation the patient developed early post-transplant diabetes and, at 21 months, she was diagnosed with Pneumocystis jirovecii pneumonia. Her basal creatinine level was 1.1 mg/dL.
The patient was admitted with a 7-day history of fever, productive cough, anorexia, right hand altered mobility and sensitivity, as well as right hand tremor and high blood pressure.
Auscultation of the lungs revealed right basal crackles and generalized wheezing. Cardiac auscultation and abdominal exam were unremarkable. Laboratory investigations revealed haemoglobin: 10 gm/dL, WBC: 15 000/mm3, neutrophils: 87%, urea: 140 mg/dL, creatinine: 4.3 mg/dL, RCP: 16 mg/dL. Urine, blood and sputum cultures were negative. Serum tacrolimus level was 20 ng/dL, 1mg twice a day prescribed, with serum level of 5 ng/dL 15 days earlier. She was on: tacrolimus 1 mg twice a day, prednisone 7.5 mg/day, micophenolate mofetil 250 mg twice a day, valganciclovir 450 mg/day, cotrimoxazol 960 mg on alternate days, darbepoetin 20 mg on alternate weeks, furosemide 40 mg/day, carvedilol 25 mg/day, omeprazole 20 mg/day and alprazolam 0.25 mg/day.
The patient was diagnosed with Aspergillus fumigates pneumonia after being submitted to bronchofibroscopic examination and bronchoalveolar lavage culture.
She was treated with voriconazol and respiratory physiotherapy, with clinical and analytical improvement.
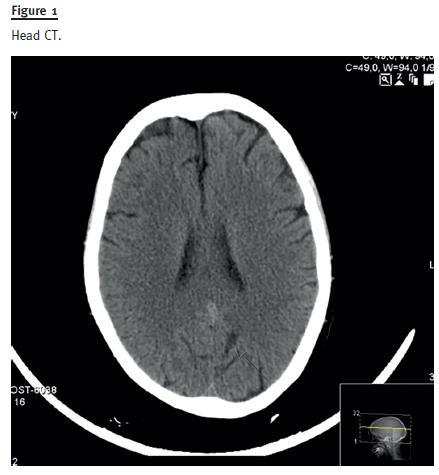
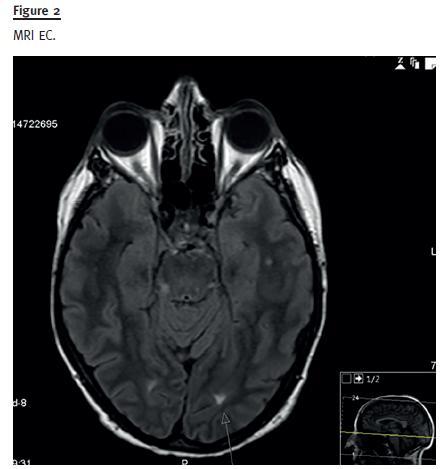
As previously noted, she also complained of altered sensation and mobility of the right hand. Electromyography and eletroencephalogram were unremarkable, but head CT revealed focal areas of hypodensity in the subcortical frontal, pre-central and internal white matter, bilaterally (Fig. 1). Further magnetic resonance (MRI) encephalocranial (EC) showed cortical hyperintense T2 lesions in the left rolandic and paramedian occipital white matter, and hypointense in T1 (Fig. 2), suggesting posterior reversible encephalopathy syndrome (PRES). As this is sometimes associated with tacrolimus toxicity, alternative calcineurin inhibitor therapy was commenced with low dose cyclosporine.
Cerebrospinal fluid, blood and urine were screened for BKV and JCV DNA, of which only the urinary sample was positive for JCV. Blood sample was also screened for EBV, CMV, herpes 1 and 2, but it was unremarkable.
There was great improvement of the neurological symptoms and control MRI-EC performed about 3 weeks later showed significant improvement compared to prior injuries.
Approximately a month later, a renal ultrasound scan showed a poorly defined cyst in the right native kidney. Further abdominal CT suggested a tumour, and the patient was submitted to right nephrectomy.
Pathology revealed clear-cell renal cell carcinoma, Fuhrman grade 2, reaching the organ capsule, but with no invasion of vascular tissue. The systematic evaluation to metastasization was negative.
After discharge, the patient has been regularly evaluated, maintaining a stable serum creatinine of 2.3 mg/dL, with no symptoms associated, namely without neurological complaints. She was on: cyclosporine 25 mg twice a day, prednisone 5 mg/day, micophenolate mofetil 250 mg twice a day, valganciclovir 450 mg/day, cotrimoxazol 960 mg on alternate days, darbepoetin 20 mg on alternate weeks, carvedilol 25 mg/day, omeprazole 20 mg/day and alprazolam 0.25 mg/day.
DISCUSSION
This clinical case highlights the potential for multiple complications associated with immunosuppressive therapy in the context of renal transplantation1-6.
This patient developed post-transplant diabetes, Aspergillus fumigatus and Pneumocystis jirovecii infections, as well as renal cell carcinoma.
She also developed PRES, a rare and less known disease. It is a syndrome of heterogeneous aetiologies that are grouped together because of similar findings on neuroimaging studies7. The pathogenesis remains unclear, but it appears to be related to abnormal cerebral autoregulation and endothelial dysfunction.
It occurs in patients on immunosuppressive and immunomodulatory therapies. The association to calcineurin inhibitors, mainly tacrolimus, occurs especially when such drugs are used in high doses8. We did not identify any pathology in the patient´s past history, namely systemic lupus erythematosus, vasculitis or other, that could be interpreted as an aetiologic factor associated to this syndrome.
The clinical syndrome is characterized by headaches, altered consciousness, visual disturbances and seizures, the latter often being the presenting manifestation in a generalized tonic clonic way. Seizures may, however begin focally and often tend to recur9.
Neuroimaging is essential to the diagnosis of PRES. Typical findings are symmetrical white matter oedema in the posterior cerebral hemispheres, particularly the parieto-occipital regions, but variations do occur. With treatment, findings on neuroimaging usually resolve within days to weeks7. A significant reduction in drug dosage or prompt removal of the cytotoxic drug is usually recommended in cases of PRES associated with cytotoxic agents. When switched to another immunosuppressive agent, patients must be monitored closely for recurrence of PRES8.
In this patient, the PRES was strongly suggested by our clinical findings, as well as the toxic serum levels of tacrolimus (even for a self-limited period of time) and brain lesions revealed by MRI. The fact that there was a significant clinical improvement following discontinuation of tacrolimus further supports this diagnosis.
CONCLUSION
Maintenance immunosuppressive therapy is administered to all renal transplant recipients to help prevent acute rejection and the loss of the renal allograft. Although an adequate level of immunosuppression is required to dampen the immune response to the allograft, the level of chronic immunosuppression is slowly decreased over time, as the risk of acute rejection decreases, to help lower the overall risk of infection, malignancy, cardiovascular disease and other possible cumulative toxicities.
This clinical case highlights the myriad of potential complications that transplant recipients are vulnerable to when receiving immunosuppressive therapy.
On the other hand, it is well known that even recommended serum levels for immunosuppressive drugs may be associated with deleterious effects, as observed in this patient.
The potential deleterious effect of transiently supra-therapeutic levels of immunosuppressant requires further research. Preventing organ rejection while subjecting patients to minimal levels of iatrogenesis remains a paramount challenge in current transplantation medicine.
References
1. Suthanthiran M, Strom TB. Renal transplantation. N Engl J Med 1994; 331(6):365-376. [ Links ]
2. Schnuelle P, Lorenz D, Trede M, Van Der Woude FJ. Impact of renal cadaveric transplantation on survival in end-stage renal failure: evidence for reduced mortality risk compared with hemodialysis during long-term follow-up. J Am Soc Nephrol 1998; 9(11):2135-2141. [ Links ]
3. Port F, Wolfe RA, Mauger EA, Berling DP, Jiang K. Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA 1993; 270(11):1339-1343. [ Links ]
4. Ojo A, Port FK, Wolfe RA, Mauger EA, Williams L, Berling DP. Comparative mortality risks of chronic dialysis and cadaveric transplantation in black end-stage renal disease patients. Am J Kidney Dis 1994; 24(1):59-64. [ Links ]
5. Apudi S, Carlin K, Bass E, Nguyen PT, Greene JN. Tacrolimus associated posterior reversible encephalopathy syndrome – a case series and review. Mediterr J Hematol Infect Dis 2014; 6; Open Journal System. [ Links ]
6. Wu Q, Marescaux C, Wolff V, et al. Tacrolimus-associated posterior reversible encephalopathy syndrome after solid organ transplantation. Eur Neurol 2010; 64(3):169-177. [ Links ]
7. Wu Q, Marescaux C, Qin X, Kessler R, Yang J. Heterogeneity of radiological spectrum in tacrolimus-associated encephalopathy after lung transplantation. Behav Neurol 2014;2014:931808. [ Links ]
8. Hammerstrom A, Howell J, Gulbis A, Rondon G, Champlin RE, Popat U. Tacrolimusassociated posterior reversible encephalopathy syndrome in hematopoietic allogenic stem cell transplantation. Am J Hematol 2013; 88(4):301-305. [ Links ]
9. Cadavid-Aljure D, Caicedo-Paredes A, Meza JC, et al. Tacrolimus associated to posterior reversible atypical encephalopaty syndrome and brain haemorrhage in renal transplant recipient. Nefrología (Madr.) 2012; 32(6): 861-863. [ Links ]
10. Ersoy A, Dizdar OS, Koc AO, Akalin H, Ener B. Aspergillus fumigatus spondylofiskitis in renal transplant patient: voriconazole experience. Exp Clin Transplant 2011; 9(4):,265-269. [ Links ]
11. Wadi J, Al-kawasmeh SI, Kamel MT, AlJayyousi BB. Disseminated invasive aspergillosis successfully treated with micafungin in a renal transplant recipient. Saudi J Kidney Dis Transpl 2010; 21(5):914-918. [ Links ]
12. Pérez-Sáez M, Mir M, Montero MM, Crespo M, et al. Invasive aspergillosis in kidney transplant recipients: a cohort study. Exp Clin Transpl 2014; 12(2):101-105. [ Links ]
13. Wadi J, Al-Kawasmeh SI, Kamel MT, AlJayyousi BB. Disseminated invasive aspergillosis successfully treated with micafungin in a renal transplant recipient. Saudi J Kidney Dis Transpl 2010; 21(5):914-918. [ Links ]
Drª Maria João Melo
Nephrology and Renal Transplantation Department,
Hospital de Santa Maria, Centro Hospitalar Lisboa Norte, EPE
Av. Prof. Egas Moniz, 1649-035 Lisboa, Portugal
E-mail: maria.melo83@gmail.com
Conflict of interest statement: None declared.
Received for publication: 20/01/2015
Accepted in revised form: 20/05/2015














