Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.29 no.1 Lisboa mar. 2015
ORIGINAL ARTICLE
Endothelial function assessed by peripheral arterial tonometry is not related with FGF23 serum levels in pre-dialysis CKD patients
A função endotelial avaliada pela tonometria arterial periférica não se correlaciona com os níveis séricos de FGF-23 em doentes renais crónicos pré-diálise
Ana Cerqueira1,2,3, Janete Quelhas-Santos2,3, Manuel Pestana1,2,3
1Department of Nephrology, São João Hospital Center, EPE. Porto, Portugal
2Department of Renal, Urological and Infectious Diseases, Faculty of Medicine, University of Porto. Porto, Portugal
3Nephrology and Infectious Diseases Research and Development Group, INEB, University of Porto. Porto, Portugal
ABSTRACT
Cardiovascular (CV) diseases are the leading causes of morbidity and mortality in patients with chronic kidney disease (CKD) that encompass the mildest degrees of renal impairment. High levels of phosphate and fibroblast growth factor 23 (FGF-23) are associated with increased CV events in this population. However, differences in clinical and pathological manifestations have suggested that distinct mechanisms may underlie cardiovascular events associated with high phosphate and FGF-23 serum levels. In animal studies endothelial dysfunction (ED) has recently been associated with increased levels of phosphorus, but not with the increase of FGF-23 serum levels. In this study, we aimed to assess endothelial function and the relationship with phosphate and FGF23 serum levels in a pre-dialysis CKD population. We examined 43 CKD patients in stages 1 to 5, followed-up in our outpatient clinic. Blood pressure, renal function, proteinuria, phosphate serum levels and Charlson Index were evaluated in the studied population. The FGF-23 levels were assessed by ELISA. Endothelial function was assessed by peripheral arterial tonometry (Endo-Pat 2000) where lower reactive hyperaemia index (RHI) values correspond to greater ED. Estimated GFR (eGFR) negatively correlated either with both serum phosphate (r = -0.42; p < 0.0004), and circulating FGF-23 levels (r = -0.42; p < 0.05); RHI positively correlated with eGFR (r = 0.35; p < 0.03) and negatively correlated with age (r = -0.59; p < 0.0001), proteinuria (r = -0.50; p < 0.03), serum phosphate (r = -0.34; p < 0.04) and Charlson index (r = -0.56; p < 0.0003). However, no significant relationship was observed between RHI and FGF23 serum levels (r = -0.11, n.s.) in the studied population. Our results suggest that peripheral arterial tonometry, a non-invasive method for evaluation of the endothelial function, can be a practical tool that adds clinically useful information to improve risk stratification in CKD predialysis patients. Our results also agree with the view that phosphate and FGF-23 serum levels might contribute to increased cardiovascular risk in CKD through distinct mechanisms.
Key-Words: Cardiovascular risk; chronic kidney disease; endothelial function; FGF-23; phosphate; tonometry.
RESUMO
As doenças cardiovasculares são a principal causa de morbilidade e mortalidade na doença renal crónica (DRC), mesmo nos estadios mais precoces. Os aumentos da fosfatemia e dos níveis séricos do factor de crescimento dos fibroblastos 23 (FGF-23) associam-se ambos a um aumento dos eventos cardiovasculares na DRC. No entanto, diferenças na tradução clínica e patológica, têm vindo a sugerir a existência de mecanismos distintos, para explicar os eventos cardiovasculares associados à hiperfosfatemia e ao aumento dos níveis séricos de FGF-23. Em estudos animais, a disfunção endotelial (DE) foi recentemente associada ao aumento dos níveis de fósforo, mas não ao aumento do FGF-23. O presente estudo avaliou a função endotelial e a sua relação com os níveis séricos de fósforo e de FGF-23 em doentes com DRC pré-diálise. Foram estudados 43 doentes com DRC incluídos nos estadios 1 a 5, seguidos em consulta externa no nosso centro. Avaliou-se a tensão arterial, a função renal, a proteinúria, os níveis séricos de fósforo e o índice de Charlson. Os níveis de FGF-23 foram avaliados por ELISA. A função endotelial foi avaliada pela tonometria arterial periférica (Endo-Pat 2000), em que a redução do índice de hiperemia reativa (RHI) corresponde a uma maior DE. A taxa de filtração glomerular estimada (eGFR) correlacionou-se negativamente, quer com a fosfatemia (r = -0.42; p < 0.,0004) quer com os níveis séricos de FGF-23 (r = -0,42; p < 0,05). O RHI correlacionou-se positivamente com a eGFR (r = 0.,35; p < 0.,04) e negativamente com a idade (r = -0,59; p < 0,0001), com a proteinúria (r = -0,50; p < 0,03), com os níveis séricos de fósforo (r = -0,34; p < 0,04) e com o índice de Charlson (r = -0,56; p < 0,0003). No entanto, não se verificou relação entre o RHI e os níveis séricos de FGF-23 (r = -0,11; ns) na população estudada. Os nossos resultados sugerem que a tonometria arterial periférica, um método não-invasivo para avaliação da função endotelial, pode constituir uma ferramenta útil na prática clínica contribuindo para estratificar o risco cardiovascular na DRC pré-diálise. Para além disso, os nossos dados apoiam a hipótese de que a influência do fósforo sérico e do FGF-23 na doença vascular pode ser mediada por mecanismos distintos.
Palavras-Chave: Doença renal crónica; função endotelial; fósforo; FGF-23; risco cardiovascular; tonometria.
INTRODUCTION
Chronic kidney disease (CKD) is strongly associated with cardiovascular disease and a graded inverse relationship between estimated glomerular filtration rate (eGFR) and cardiovascular event rates has emerged from large-scale observational studies1. As cardiovascular diseases are the leading cause of death in CKD patients, it is of great importance to have instruments to stratify cardiovascular risk in these populations2. Although classic cardiovascular risk factors are almost universally present in CKD, they cannot account for the increased CV risk in these patients3.
Disturbances in phosphate homeostasis occur in most CKD patients and growing evidence suggests that hyperphosphatemia is associated with increased all-cause mortality and cardiovascular events in this population. Hyperphosphatemia correlates with the calcification of coronary arteries and cardiac valves. In addition, in vitro studies have shown that phosphate induces vascular calcification and accelerates the osteogenic transformation of vascular smooth muscle cells4,5.
Fibroblast growth factor 23 (FGF-23) is a circulating endocrine hormone that is secreted by osteocytes, primarily in response to increases in dietary phosphate intake6. The classic actions of FGF-23 on mineral metabolism are mediated by binding of FGF-23 to heterodimeric complexes consisting of FGF receptors and the specific FGF-23 co-receptor, Klotho7. Activation of Klotho-FGF receptor complexes in the kidney augments urinary phosphate excretion and decreases 1.25 dihydroxyvitamin D synthesis8. Notwithstanding the desirable effects of FGF-23 to maintain phosphate homeostasis in CKD, a growing body of evidence supports the view that high FGF-23 serum levels are also a risk factor for cardiovascular disease in these populations7. However, it has been suggested that distinct mechanisms may underlie the increased rate of cardiovascular events associated with high phosphate and FGF-23 serum levels in CKD. Indeed, animal models implicate high serum phosphate as mechanism of vascular calcification and endothelial dysfunction, whereas high levels of FGF-23 have been implicated in left ventricular hypertrophy through mechanisms distinct from activation of Klotho-FGF receptor9.
Endothelial dysfunction (ED) represents the earliest abnormality in the development of vascular disease linked to atherosclerosis. Pulse tonometry is a rapid, non-invasive and non-operator-dependent technique to assess endothelial vasodilator function10 and peripheral endothelial function assessed by Endo-PAT was found to be an independent predictor of cardiovascular events in CKD patients10,11.
To the extent that the increase in serum phosphorus levels but not high levels of FGF-23 serum levels have been implicated as mechanism of ED in CKD12,13, we aimed to examine endothelial function using Endo-PAT in a pre-dialysis CKD population and the relationship with both FGF-23 and phosphate serum levels.
METHODS
We examined 43 CKD patients followed-up in our outpatient clinic of the Nephrology department of the Centro Hospitalar de São João. The patients were distributed according to the GFR calculated by CKD-EPI formula (stage 1: > 90ml/min/1.73m2; stage 2: between 60-89ml/min/1.73m2; stage 3: between 30-59ml/min/1.73m2; stage 4: between 15-29 ml/min/1.73m2; stage 5: < 15 ml/min/1.73m2). Patients were studied in standard conditions (no changes were made in their usual medication, nor have extra reinforcement of diet restrictions or smoke abstinence been done). The patients were randomly selected, without previous knowledge, in their routine appointment, after being informed about the purpose and methods of the study giving voluntarily their written informed consent. Patients with arteriovenous fistula (which prevents evaluation with Endo-PAT), acute kidney injury, recent hospital admission (< 2 weeks), recent infections (< 1 week) and known psychiatric disturbances were excluded from the study.
The research was approved by the Ethics Committee for Health and the Local Institutional Review Board of São João Hospital Centre, and was carried out in accordance with the Declaration of Helsinki 2008 of the World Medical Association.
Blood and urine samples were collected in all participants. Blood pressure, renal function, proteinuria, phosphate serum levels and a validated comorbidity index (Charlson Index) were evaluated in the studied population. Intact FGF-23 levels were assessed by an ELISA kit (Immutopics, Inc.).
Endothelial function was assessed by peripheral arterial tonometry (Endo-Pat 2000). The Endo-Pat serve as a measure of peripheral vasomotor function, in which nitric oxide has shown to be an important contributor14 as was validated in the large, community-based Framingham study15. The technique provides values for the calculation of a reactive hyperaemia index (RHI), which gives an indication of the endothelial vasodilator function.
The RHI is the post-to-pre occlusion PAT signal ratio in the occluded arm, relative to the same ratio in the control arm, and corrected for baseline vascular tone11. Lower RHI values correspond to greater ED.
STATISTICS
All data are presented as means ± SEM. A p < 0.05 was assumed to denote a significant difference.
For group comparisons, one-way ANOVA followed by post hoc tukey test or standard unpaired student´s t-test was used when appropriate. Pearson correlation was used to evaluate the relationship between two continuous variables and a two-tailed p is presented.
Data were analysed using Microsoft Excel 2010 (WA, USA) and GraphPad Prism 5 (CA, USA) software. Multivariate linear regression was used to investigate the association between Charlson Index, serum phosphate, FGF-23, RHI and age with eGFR (CKD-Epi). In all analyses, a p-value of < 0.05 was considered as statistically significant. Statistical analyses were conducted using R software version 2.14.1 (Project for Statistical Computing).
RESULTS
The demographic and clinical characteristics of the studied population are presented in Table 1. The patients were distributed according to the eGFR calculated by CKD-EPI formula as: CKD stages 1-2 [n = 16, M37%, age 48.6 ± 3.2], CKD stage 3 [n = 14, M33%, age 58.0 ± 4.1], CKD stages 4-5 [n = 13, M33%, age 59.8 ± 4.0]. Demographic characteristics only achieved statistically significant differences concerning patients age [CKD stages 1-2 vs. 5-4 (48.6 ± 3.2 vs. 59.8 ± 4.0, p.<.0.05)]. No significant differences were observed among the three groups regarding CKD aetiology, prescribed antihypertensive drugs, body mass index (BMI), diabetes (DM) and hypertension prevalences. As expected, the Charlson Index was significantly higher in stages 4-5 group than in stages 1-2 group (5.3 ± 0.6 vs. 1.8 ± 0.6; p < 0.05).
The biochemical and other analytical parameters of the studied population are presented in Table 2.
As expected, we found statistically significant differences among the three groups, regarding haemoglobin level, urinary protein/creatinine ratio, serum phosphate levels, iPTH, FGF-23, uric acid and brain natriuretic peptide.
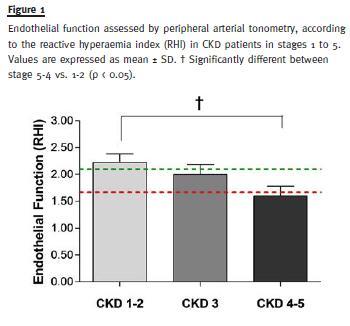
Concerning endothelial function assessed by Endo-Pat, RHI was found significantly lower in CKD stages 4-5 in comparison with CKD stages 1-2 (1.60 ± 0.18 vs. 2.22 ± 0.16, p < 0.05) (Fig. 1). In unadjusted analysis, RHI positively correlated with eGFR (r = 0.35; p < 0.04) and negatively correlated with age (r = -0.59; p < 0.0001), proteinuria (r = -0.50; p < 0.03), phosphate serum levels (r = -0.34; p < 0.04) and Charlson index (r = -0.56; p < 0.0003) (Fig. 2).
In addition, in unadjusted analysis eGFR negatively correlated with FGF-23 (r = -0.42; p < 0.05), phosphate serum levels (r = -0.42; p < 0.0004), Charlson index (r = -0.63; p < 0.0001) and age (r = -0.45; p < 0.003) (Fig. 2). In the multivariate analysis, the relationships of eGFR with both phosphate serum levels and FGF-23 persisted after adjustment for demographic and clinical variables associated with reduced renal function (Table 3).
No significant relationship was observed between RHI and FGF-23 in the studied population even in unadjusted analysis (r = -0.11, n.s.) (Fig. 2).
DISCUSSION
The search for biomarkers that could explain or characterize the enhanced CV risk in CKD patients is of utmost importance and has gained prominence in the last few years. In relation with this subject, ED has been proposed as an early event of pathophysiological relevance in the atherosclerotic process that relates to higher risk of CV events in CKD1. In the present study, carried out in a pre-dialysis CKD population, we confirmed that the decrease of renal function is associated with increased ED16. In addition, we found in our predialysis CKD patients that ED assessed by pulse tonometry also correlated positively with age, high scores of Charlson comorbidity index, increased proteinuria and high levels of serum phosphate. Since ED can be reversed or improved by lifestyle modifications18 and medical therapies19, our results suggest that pulse tonometry, a non-invasive method for evaluation of ED, may add clinically useful information to improve risk stratification in CKD pre-dialysis patients17, thus contributing to better follow-up of this population20,21.
Another interesting finding of our study is that, in contrast to serum phosphate levels, circulating FGF-23 levels were not associated with ED. This dissociation, when viewed collectively with the observations that implicate circulating FGF-23 levels in the increased CV risk and mortality in CKD, lends further support to the view that targeting FGF-23 may not be useful in the prevention and management of uraemic vascular disease.
Our results fit well with recent findings in animal studies showing that incubation of cultured endothelial cells with high phosphate concentrations induces apoptosis, stimulates production of reactive oxygen species and impairs secretion of NO in response to acetylcholine22, whereas the effect linking higher levels of FGF-23 with cardiovascular disease is independent of serum phosphate levels and mainly implicates the development of left ventricular hypertrophy7. This study has the virtue of extrapolating to humans, the recent observations in animal studies that suggested that FGF-23 and serum phosphate promote distinct mechanisms of cardiovascular toxicity7. Further investigation is needed to confirm these data and explore the pathophysiological mechanisms that underlie the increased cardiovascular risk associated with high phosphate and FGF-23 serum levels in CKD.
We acknowledge some limitations of our study. Firstly, those inherent to the observational nature of the study and the limited number of patients included, particularly in the more advanced CKD stages. Second, the assessment of endothelial function by pulse tonometry has an intrinsic limitation in patients with more advanced CKD stages because many of those patients already have an arteriovenous fistula, according to their pre-dialysis preparation, which compromises the assessment of endothelial function with Endo-Pat. Another limitation of our study is that patients with more advanced CKD stages (4 and 5) were significantly older than those included in stages 1- 2. Although we recognize that this may be a confounding factor, namely because age contributes to the decrease of renal function as well as to the increase of cardiovascular events, this does not preclude our conclusion that there is no relationship between FGF-23 and ED in pre-dialysis CKD patients, even in univariate analysis.
ACKNOWLEDGEMENTS
Supported by a clinical research grant from Sociedade Portuguesa de Nefrologia (project titled Human renalase: relationship with mechanisms involved in increased cardiovascular risk in chronic kidney disease) and PEst-OE/SAU/UI0725/2014 from FCT/COMPETE/FEDER.
References
1. Moody WE, Edwards NC, Madhani M, et al. Endothelial dysfunction and cardiovascular disease in early-stage chronic kidney disease: Cause or association? Atherosclerosis 2012;223(1):86–94. [ Links ]
2. Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003;108(17):2154–2169. [ Links ]
3. Zoccali C. Cardiovascular risk in uraemic patients-is it fully explained by classical risk factors? Nephrol Dial Transplant 2000;15(4):454–457. [ Links ]
4. Ma KL, Liu J, Ni J, et al. Inflammatory stress exacerbates the progression of cardiac fibrosis in high-fat-fed apolipoprotein E knockout mice via endothelial-mesenchymal transition. Int J Med Sci 2013;10(4): 420–426. [ Links ]
5. Hruska K, Mathew S, Lund R, Fang Y, Sugatani T. Cardiovascular risk factors in chronic kidney disease: does phosphate qualify? Kidney Int Suppl 2011;79(121):S9–S13. [ Links ]
6. Saji F, Shigematsu T, Sakaguchi T, et al. Fibroblast growth factor 23 production in bone is directly regulated by 1{alpha±, 25-dihydroxyvitamin D , but not PTH. Am J Physiol Renal Physiol 2010;299(5)F1212–1217. [ Links ]
7. Scialla JJ, Wolf M. Roles of phosphate and fibroblast growth factor 23 in cardiovascular disease. Nat Rev Nephrol 2014;10(5):268–278. [ Links ]
8. Hu MC, Shiizaki K, Kuro-o M, Moe OW. Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol 2013;75:503–533. [ Links ]
9. Shalhoub V, Shatzen EM, Ward SC, et al. FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J Clin Invest 2012;122(7):2543–2553. [ Links ]
10. Hirata Y, Sugiyama S, Yamamoto E, et al. Endothelial function and cardiovascular events in chronic kidney disease. Int J Cardiol 2014;173(3):481–486. [ Links ]
11. Axtell AL, Gomari FA, Cooke JP. Assessing endothelial vasodilator function with the Endo-PAT 2000. J Vis Exp 2010;(44).pii:2167. doi: 10.3791/2167. [ Links ]
12. Lim K, Lu TS, Molostvov G, et al. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation 2012;125(18):2243–2255. [ Links ]
13. Medici D, Razzaque MS, Deluca S, et al. FGF-23-Klotho signaling stimulates proliferation and prevents vitamin D-induced apoptosis. J Cell Biol 2008;182(3):459–465. [ Links ]
14. Nohria A, Gerhard-Herman M, Creager MA, Hurley S, Mitra D, Ganz P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol (1985) 2006;101(2):545–548. [ Links ]
15. Hamburg NM, Keyes MJ, Larson MG, et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation 2008;117(19):2467–2474. [ Links ]
16. Stam F, van Guldener C, Schalkwijk CG, ter Wee PM, Donker AJ JM, Stehouwer CD. Impaired renal function is associated with markers of endothelial dysfunction and increased inflammatory activity. Nephrol Dial Transplant 2003;18(5):892–898. [ Links ]
17. Rubinshtein R, Kuvin JT, Soffler M, et al. Assessment of endothelial function by noninvasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J 2010;31(9):1142–1148. [ Links ]
18. Matsuzawa Y, Sugiyama S, Sugamura K, et al. Successful diet and exercise therapy as evaluated on self-assessment score significantly improves endothelial function in metabolic syndrome patients. Circ J 2013;77(11):2807–2815. [ Links ]
19. Tonetti MS, DAiuto F, Nibali L, et al. Treatment of periodontitis and endothelial function. N Engl J Med 2007;356(9):911–920. [ Links ]
20. Kitta Y, Obata J, Nakamura T, et al. Persistent impairment of endothelial vasomotor function has a negative impact on outcome in patients with coronary artery disease. J Am Coll Cardiol 2009;53(4):323–330. [ Links ]
21. Matsubara J, Sugiyama S, Akiyama E, et al. Dipeptidyl peptidase-4 inhibitor, sitagliptin, improves endothelial dysfunction in association with its anti-inflammatory effects in patients with coronary artery disease and uncontrolled diabetes. Circ J 2013;77(5):1337–1344. [ Links ]
22. Peng A, Wu T, Zeng C, et al. >Adverse effects of simulated hyper- and hypo-phosphatemia on endothelial cell function and viability. PLoS One 2011;6(8):e23268. [ Links ]
Drª Ana Cerqueira
Department of Nephrology
São João Hospital Center, EPE
Alameda Prof. Hernâni Monteiro
4200-319, Porto, Portugal.
E-mail: anacerqueira-1@hotmail.com
Conflict of interest statement: All the authors declare that there is no conflict of interest.
Received for publication: 12/07/2014
Accepted in revised form: 04/02/2015














