Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.29 no.1 Lisboa mar. 2015
ORIGINAL ARTICLE
Conversion from twice-daily tacrolimus to an extended release formulation in stable kidney transplant recipients: 2-year follow-up results of a single centre
Conversão de tacrolimus de duas tomas diárias para uma formulação de libertação prolongada em doentes transplantados renais estáveis: resultados do follow-up de 2 anos de um centro
Luis Rodrigues1, Fernando Macario1, Catia Pego2, Marta Neves1, Catarina Romaozinho1, Lidia Santos1, Rui Alves1, Helena Sa1, Alfredo Mota3, Mario Campos1
1Department of Nephrology, Centro Hospitalar e Universitário de Coimbra. Coimbra, Portugal
2Department of Nephrology, Hospital de São Teotónio. Viseu, Portugal
3Department of Urology and Renal Transplantation, Centro Hospitalar e Universitário de Coimbra. Coimbra, Portugal
ABSTRACT
Introduction: Calcineurin inhibitors are currently considered the cornerstone of maintenance immunosuppression in renal transplantation. The extended release formulation of tacrolimus (ER-TAC) was developed to improve drug adherence in these patients. The long-term safety and efficacy of the conversion from the twice-daily tacrolimus to the ER-TAC is still undetermined. Subjects and Methods: Retrospective registry-based single centre study including all renal transplant recipients converted from TAC twice-daily to the ER-TAC on a 1: 1 mg basis. We collected biometric data, induction regimens, acute rejection episodes and death. Variables of interest [serum creatinine, blood urea nitrogen, fasting glucose, urinalysis, haemoglobin, TAC dose (mg/kg) and TAC trough levels (ng/ml)] were registered at conversion and 1, 6, 12 months and at last follow-up post-conversion. Results: We analysed 127 patients, 71.9 % male, mean age at conversion (± SD) was 49.8 ± 13.6 years. Conversion occurred at 4.10 ± 3.83 years after transplant and our mean follow-up time was 2.56 ± 0.55 years. TAC trough levels (ng/ml) progressively decreased from pre-conversion to the following stages (pre-conversion: 7.41 ± 2.61 vs. last followup: 5.04 ± 1.86, p < 0.001) but no significant dose adjustments were necessary for attaining predetermined target levels. Renal function remained stable and there were no significant differences in glucose metabolism pre and post conversion. No significant adverse effects were verified. There were no episodes of acute rejection throughout the follow-up. Conclusions: The conversion to ER-TAC ensured effective immunosuppression over a follow-up of 2 years suggesting that this formulation is an excellent alternative to the conventional one.
Key-Words: Conversion; extended release; immunosuppression; renal transplantation; tacrolimus.
RESUMO
Introdução: Os inibidores da calcineurina são atualmente considerados a pedra basilar da imunossupressão de manutenção na transplantação renal. A formulação de libertação prolongada do tacrolimus (ER-TAC) foi desenvolvida para melhorar a aderência farmacológica destes doentes. A segurança e a eficácia em longo prazo da conversão do tacrolimus de duas tomas diárias para a ER-TAC permanecem indeterminadas. Material e métodos: Estudo retrospectivo de centro único que incluiu todos os doentes transplantados renais convertidos de TAC de duas tomas diárias para a ER-TAC na proporção de 1: 1 mg. Recolhemos dados biométricos, regimes de indução, episódios de rejeição aguda e morte. Variáveis de interesse (creatinina sérica, azoto ureico, glicose em jejum, análise de sedimento, hemoglobina, dose de TAC (mg/Kg) e níveis de TAC (ng/ml)) foram registados na conversão e aos 1, 6, 12 meses e no último follow-up pós-conversão. Resultados: Analisamos 127 doentes, 71.9% homens, idade média na conversão (± DP) 49.8 ± 13.6 anos. A conversão ocorreu aos 4.10 ± 3.83 anos pós-transplante e o nosso follow-up médio foi de 2.56 ± 0.55 anos. Os níveis de TAC desceram progressivamente desde a pré-conversão para as observações seguintes (pré-conversão: 7.41 ± 2.61 vs último follow-up: 5.04 ± 1.86, p<0,001) mas não foram necessários ajustes de dose significativos para atingir os níveis alvo pré-definidos. A função renal permaneceu estável e não ocorreram diferenças significativas no metabolismo da glicose pré e pós-conversão. Não foram verificados efeitos adversos significativos. Não ocorreram episódios de rejeição aguda durante o follow-up. Conclusão: A conversão para a ER-TAC proporcionou uma imunossupressão eficaz durante um follow-up de 2 anos sugerindo que esta formulação é uma excelente alternativa à convencional.
Palavras-Chave: Conversão; libertação prolongada; imunossupressão; tacrolimus; transplantação renal.
INTRODUCTION
The need for maintenance immunosuppressive therapy in renal transplantation was soon found indispensable for the prevention of acute rejection and the loss of the renal allograft. The optimal therapy in these patients is still under debate but the role of calcineurin inhibitors (CNI) seems crucial and most transplant centres utilize a regimen with these agents1. Tacrolimus (TAC) is currently considered the first-line CNI for renal transplantation2.
Several studies have showed superior graft function, better prevention of acute rejection and superior graft survival with comparison to cyclosporine3,4. Nonadherence is one of the most important risk factors for allograft loss over the long-term and the odds of graft failure are reported to be increased sevenfold in these patients5. A recent comparison of kidney graft survival rates between Europe and the United States (US) pointed out that non-adherence issues6 could explain the lower long-term kidney graft survival found in the US. In 2007, an extended release Tacrolimus (ER-TAC) formulation (Advagraf®; Astellas Pharma Inc.) was designed in order to improve adherence by providing a more convenient once-daily dosing. Pharmacokinetic phases I and II studies showed equivalence between the ER-TAC and the twice-daily standard release TAC (BID-TAC)7. Clinical experience in current practice with the conversion from
BID-TAC to an ER formulation has been described in several observational studies with excellent results demonstrated with relation to acute rejection rate and other efficacy and safety considerations8-11. The follow-up of these reports was limited to a maximum of twelve months. Further evaluation is required to access the long-term effects of this conversion.
We aimed to analyse the safety and efficacy of conversion from BID-TAC to an ER formulation in stable kidney transplant recipients, reporting our experience over a longer period.
SUBJECTS AND METHODS
We performed a retrospective registry-based single centre study of all renal transplant recipients treated with BID-TAC (Prograft®; Astellas Pharma Inc.) and converted to the ER formulation on a 1: 1 mg basis. According to our practice, these patients were only converted if in a stable clinical condition defined as a minimum 6-month post-transplant status, absence of an acute clinical hazardous event (cardiovascular, infectious or rejection episode) and absence of a significant increase (> 0.3 mg/dL) in serum creatinine in the previous 3 months. Patients included attained a pre-conversion TAC-trough level that is within our target values adjusted to transplant time (during the first three months 8-12 ng/ml, during the following year 7-10 ng/ml and thereafter 4-8 ng/ml). Our current induction therapy consists of an anti-IL-2 receptor antibody (Basiliximab) for lower immunological risk patients and a polyclonal anti-lymphocyte antibody for higher risk patients (Re-transplantation, elevated panel-reactive antibody, black-race, younger recipient age and cold ischaemia time > 24 hours). Our standard maintenance immunosuppression regimen consists of triple therapy with the CNI, prednisone and an antimetabolite (mycofenolate mofetil or mycophenolic acid). This therapy is progressively adjusted to the transplant time and the patients immunological risk according to evidence-based clinical practices.
Conversion started in June 2009 and our reported follow-up leads up to October 2012. We collected biometric variables (body weight, blood pressure), induction and maintenance immunosuppression regimens, acute rejection episodes and death. Laboratory variables of interest (serum creatinine (sCr), blood urea nitrogen, fasting glucose, urinalysis, haemoglobin, TAC dose (mg/kg) and TAC trough levels (ng/ml) were registered pre-conversion and at 1, 6, 12 months and at last follow-up post-conversion. Glomerular filtration rate (GFR) was estimated from serum creatinine using the Modification of Diet in Renal Disease Study (MDRD-4) equation12. We also reviewed reported side-effects and drug discontinuation motives.
Our primary endpoints were patient and graft survival. Secondary endpoints included episodes of acute rejection and graft function and glucose metabolism evolution.
Statistical analysis was performed using statistical package SPSS 17.0 (SPSS Inc., Chicago, IL, USA) and significance was assessed at the two-tailed 0.05 threshold.
For continuous variables means ± standard deviations (SD) are given and for categorical variables frequency and percentages of patients are shown. The paired students t-tests or Wilcoxon signed-rank tests, as appropriate, were applied for the study of continuous variables and the Chi-squared test or the Fishers exact test were applied in the categorical variables as appropriate.
RESULTS
The analysis included 127 patients (71.9% male). Mean age at conversion was 49.8 ± 13.6 years, mean time from transplantation to conversion was 4.10 ± 3.83 years and our mean follow-up time was 2.56 ± 0.55 years. The patients were transplanted between September 1987 and November 2010 with 10.2% being re-transplantations (second transplant in all), living donor in 7.1% and expanded criteria donor in 43.3%.
Twenty-one (16.5%) were considered higher immunological risk patients and received induction therapy with a polyclonal anti-lymphocyte antibody. The aetiology of chronic kidney disease was undetermined in 39.3%, and the main determined causes were chronic glomerulonephritis in 22.0%, autosomal dominant polycystic kidney disease in 12.5 %, chronic interstitial nephritis in 10.2%, hypertension in 7.8% and diabetes in 7.0%.
The mean TAC dose (mg/Kg/day) at conversion was 0.067 ± 0.037 and 12 months post-conversion 0.065 ± 0.032, p = 0.33. At the last follow-up TAC dose significantly decreased to 0.060 ± 0.031, p = 0.004. The TAC trough levels (ng/ml) significantly decreased from pre-conversion to the following stages: pre-conversion: 7.41 ± 2.61, twelve months 6.21 ± 2.50 and at the last follow-up 5.04 ± 1.86, p < 0.001 (Fig. 1). The mean change of these levels at 12 months was -15.7% and at the last follow-up – 31.9%. At conversion, 98.4% of patients were also treated with prednisone and 88.1% with an antimetabolite. At the last follow-up, no important changes were observed in concomitant immunosuppression.
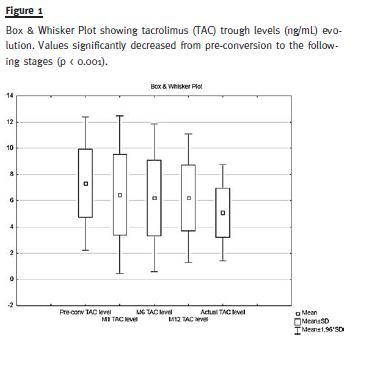
Regarding our primary endpoints, we report no deaths throughout the follow-up. Post-conversion graft survival at 12 and 24 months was 100%. One patient returned to dialysis 29 months after conversion due to advanced biopsy-proven interstitial fibrosis and tubular atrophy.
Mean GFR values did not change significantly during follow-up (69.87 ± 23.8 mL/min at conversion versus 71.29 ± 28.7 mL/min at last follow-up, p = 0.105). (Fig. 2)
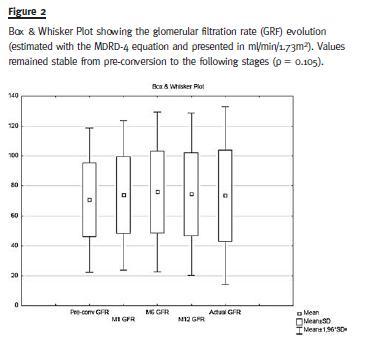
There were no episodes of acute rejection throughout the observed period. There were also no significant differences found in fasting serum glucose, haemoglobin, proteinuria and blood-pressure values pre- and post-conversion. Of the eleven patients diagnosed with new-onset diabetes after transplant only two (1.57%) ensued after conversion.
Discontinuation of ER-TAC occurred in three patients (2.36%): two patients were diagnosed with malignancy (skin and colon) and one patient had suspected CNI-related nephrotoxicity and was converted to an m-TOR inhibitor. No other significant adverse effects were verified.
DISCUSSION
Our centre started the conversion of transplant recipients treated with BID-TAC to the ER-TAC formulation aiming to improve drug adherence. With our practice supported by clinical evidence, we find this to be a key element in the prevention of allograft loss over the long-term5. Our results support that the conversion of stable renal transplant recipients treated with BID-TAC to the ER-TAC formulation is safe and efficient up to a medium follow-up of 2 years. To this date, the largest study assessing the safety and efficacy of conversion from BID-TAC to the ER-TAC in maintenance kidney transplant recipients study was restricted to 12-months11. This large Spanish multicentre, prospective, observational study (GREAT Study Group) published their results in 2011 and reported that the conversion provided stable renal function, a low acute rejection rate, and good tolerability in stable kidney transplant recipients in the routine clinical practice setting. Several other studies have reported similar results comprehensive to the same maximum follow-up time7,8,10.
Our analysis is in concordance with published results regarding a progressive TAC trough level decrease after conversion8,9,11,13. Some authors opted by increasing their TAC dosage to compensate for this finding8,10,13.
In our study, the mean level change at 12 months of -15.7% was accepted since those values remained within our targets and adjusted to the patient´s transplant time. We significantly reduced the patients TAC exposure after the 12 months, according to our centre practices.
The unexpected decrease in TAC trough levels has already been questioned regarding a possible subclinical immunological graft damage that could compromise lifelong survival versus a possible benefit in relation with reduced nephrotoxicity14. The results of the Symphony study, a large multicentre randomized control study in de novo kidney transplant recipients (n = 1645) showed superior graft function, better prevention of acute rejection and superior graft survival at 12 months with daclizumab induction and low-dose tacrolimus (C0 3–7 ng/ml)15. Although Prograft® was used in that study, we consider that we can possibly expect the same results with the use of the ER formulation and the addressed targets of TAC though levels. Although we found no significant need for dose adjustment after conversion to compensate for the TAC trough level decrease, regular monitoring is advised especially if the conversion is performed early after transplantation when higher targets are in order. This is in agreement with the 2009 KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients2.
Our study has several limitations inherent to its retrospective registry-based single centre design. Conversion to ER-TAC was performed in a selected method, excluding patients with a higher risk for acute rejection during at least 6 months after transplantation, such as a previous acute rejection episode and/or other hazardous events. We, therefore, cannot expand our conclusions to other transplant recipients. Protocol biopsies are actually the only method to detect subclinical rejection and there is evidence of its association with chronic allograft injure and reduced graft survival16,17. Although we cannot exclude this form of injury, our excellent and stable estimated GFR during the follow-up stands against it. We also do not have an independent measure of improved drug adherence with the ER formulation, but there is compelling evidence that reducing dosing frequency can improve adherence and graft outcome18. Randomized control trials are still needed to test interventions to improve adherence in kidney transplant patients.
In conclusion, our study demonstrated the maintenance of effective immunosuppression in patients converted to ER-TAC over a follow-up of more than 2 years, suggesting this to be an excellent alternative to the conventional formulation.
References
1. Wong W, Venetz JP, Tolkoff-Rubin N, Pascual M. 2005 immunosuppressive strategies in kidney transplantation: which role for the calcineurin inhibitors? Transplantation 2005;80(3):289-96. [ Links ]
2. Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2009;9 Suppl 3:S1-155. [ Links ]
3. Webster AC, Woodroffe RC, Taylor RS, Chapman JR, Craig JC. Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: meta-analysis and meta-regression of randomised trial data. BMJ 2005;331(7520):810. [ Links ]
4. Kramer BK, Montagnino G, Del Castillo D, et al. Efficacy and safety of tacrolimus compared with cyclosporin A microemulsion in renal transplantation: 2 year follow-up results. Nephrol Dial Transplant 2005;20(5):968-73. [ Links ]
5. Butler JA, Roderick P, Mullee M, Mason JC, Peveler RC. Frequency and impact of nonadherence to immunosuppressants after renal transplantation: a systematic review. Transplantation 2004;77(5):769-76. [ Links ]
6. Gondos A, Döhler B, Brenner H, Opelz G. Kidney graft survival in Europe and the United States: strikingly different long-term outcomes. Transplantation 2013;95(2):267-74. [ Links ]
7. Chisholm MA, Middleton MD. Modified-release tacrolimus. Ann Pharmacother 2006; 40(2):270-5. [ Links ]
8. Hougardy JM, Broeders N, Kianda M, et al. Conversion from prograf to advagraf among kidney transplant recipients results in sustained decrease in tacrolimus exposure. Transplantation 2011;91(5):566-9. [ Links ]
9. Wu SW, Tsai HC, Tsai PY, Hung TW, Chang HR, Lian JD. Conversion to prolonged release tacrolimus formulation in stable kidney transplant recipients. Swiss Med Wkly 2013;143:w13850. [ Links ]
10. Meçule A, Poli L, Nofroni I, et al. Once daily tacrolimus formulation: monitoring of plasma levels, graft function, and cardiovascular risk factors. Transplant Proc 2010;42(4):1317-9. [ Links ]
11. Guirado L, Cantarell C, Franco A, et al. with the GREAT Study Group. Efficacy and safety of conversion from twice-daily to once-daily tacrolimus in a large cohort of stable kidney transplant recipients. Am J Transplant 2011;11(9):1965-71. [ Links ]
12. Levey AS, Coresh J, Greene T, et al. with the Chronic Kidney Disease Epidemiology Collaboration. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 2007;53(4):766-72. [ Links ]
13. de Jonge H, Kuypers DR, Verbeke K, Vanrenterghem Y. Reduced C0 concentrations and increased dose requirements in renal allograft recipients converted to the novel once-daily tacrolimus formulation. Transplantation 2010;90(5):523-9. [ Links ]
14. Hougardy JM, de Jonge H, Kuypers D, Abramowicz D. The once-daily formulation of tacrolimus: a step forward in kidney transplantation? Transplantation 2012;93(3):241-3. [ Links ]
15. Ekberg H, Tedesco-Silva H, Demirbas A, et al. with the ELITE-Symphony Study. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 2007;357(25):2562-75. [ Links ]
16. Shishido S, Asanuma H, Nakai H, et al. The impact of repeated subclinical acute rejection on the progression of chronic allograft nephropathy. J Am Soc Nephrol 2003;14(4):1046-52. [ Links ]
17. Nankivell BJ, Borrows RJ, Fung CL, OConnell PJ, Allen RD, Chapman JR. Natural history, risk factors, and impact of subclinical rejection in kidney transplantation. Transplantation 2004;78(2):242-9. [ Links ]
18. Takemoto SK, Pinsky BW, Schnitzler MA, et al. A retrospective analysis of immunosuppression compliance, dose reduction and discontinuation in kidney transplant recipients. Am J Transplant 2007;7(12):2704-11. [ Links ]
Dr. Luís Rodrigues
Department of Nephrology, Centro Hospitalar e Universitário de Coimbra. Coimbra, Portugal
Praceta Professor Mota Pinto 3000-075 Coimbra, Portugal.
E-mail: luis.arodrigues@hotmail.com
End page: This work had no support in the form of grants, equipment,drugs, or any combination thereof.
Conflict of interest statement: None declared.
Received for publication: 05/01/2015
Accepted in revised form: 29/01/2015














