Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Portuguese Journal of Nephrology & Hypertension
Print version ISSN 0872-0169
Port J Nephrol Hypert vol.28 no.4 Lisboa Dec. 2014
ORIGINAL ARTICLE
Renal impairment at presentation is not an adverse prognostic factor in multiple myeloma patients treated with new agents
Insuficiência renal à apresentação não é um factor prognóstico adverso em doentes com mieloma múltiplo tratados com novos agentes
Manuel Neves, Helena Martins, Graca Esteves, Eduardo Espada, Isabel Pereira, Carlos Martins, Jose Alves Do Carmo
Department of Haemathology and Bone Marrow Transplantation, Centro Hospitalar Lisboa Norte –
ABSTRACT
Fifteen to forty per cent of multiple myeloma patients have renal impairment at diagnosis. The Durie-Salmon staging included renal impairment as an adverse prognostic factor and so does the International Staging System, since β2-microglobulin correlates with both tumour burden and renal impairment. However, the introduction of new therapeutic agents (as bortezomib, lenalidomide, and thalidomide) and new prognostic factors, such as cytogenetics, changed that view. With this paper, we intended to study the prognostic impact of renal insufficiency at presentation in patients with multiple myeloma treated with new agents. We retrospectively analysed, from July 2004 to December 2012, multiple myeloma patients treated in first line with bortezomib or thalidomide, in whom cytogenetic study was performed. Renal insufficiency was defined as creatinine clearance < 30mL/min, using the Cockcroft-Gault equation. We treated 174 patients, 54 with thalidomide (31%), 120 with bortezomib (69%). Eighty-eight patients were submitted to autologous stem-cell transplantation. Male sex = 50%, median age = 66 years (22-83).
There were 13.4% of light chain myelomas. Creatinine clearance < 30mL/min in 20.1% of patients. International Staging System: I = 39.1%; II = 28.7% and III = 32.2%. High-risk cytogenetics = 35.1%. Median follow-up = 30 months, median overall survival = 83 months. In univariate analysis, the median overall survival was higher for patients without renal impairment compared to patients with renal impairment (83 vs. 40 months, p = 0.004). However, in multivariate analysis with the International Staging System and high-risk cytogenetics, renal impairment does not affect the overall survival. We concluded that renal impairment at presentation is not a prognostic factor in multiple myeloma patients treated in first line with new therapeutic agents.
Key Words: Autologous stem cell transplantation; bortezomib; multiple myeloma; renal impairment; thalidomide.
RESUMO
Quinze a 40% dos doentes com mieloma multiplo apresentam-se com insuficiencia renal ao diagnostico.
O estadiamento de Durie-Salmon considerava a insuficiencia renal factor de mau prognostico e, actualmente, tambem o International Staging System, ja que a β2-microglobulina se correlaciona tanto com a cargatumoral como com a insuficiencia renal. Contudo, a introducao de novos agentes (como bortezomib, lenalidomida e talidomida) e o aparecimento de outros factores de prognostico, como a citogenetica, mudaram esse panorama. Pretendeu-se, com este estudo, caracterizar a importancia prognostica da insuficiencia renal a apresentacao nos doentes com mieloma multiplo tratados com novos agentes. Analisamos retrospectivamente, entre Julho 2004 e Dezembro 2012, todos os doentes com mieloma multiplo de novo tratados em primeira linha com bortezomib ou talidomida e com estudo citogenetico. Insuficiencia renal caracterizada como clearance de creatinina < 30mL/min, calculada pela equacao de Cockcroft-Gault. Foram tratados 174 doentes, 54 com talidomida (31%)120 com bortezomib (69%), 88 dos quais seguiram depois para auto transplante. Sexo masculino = 50% e mediana de idades = 66 anos (22-83). Mieloma multiplo de cadeias leves em 13,4%. Clearance creatinina < 30mL/min em 20,1% dos doentes. International Staging System: I = 39,1%; II = 28,7% e III = 32,2%. Citogenetica de alto risco = 35,1%. Mediana de follow-up 30 meses, mediana de sobrevivencia global 83 meses. Em analise univariada constata-se diferenca estatisticamente significativa entre a sobrevivencia global dos doentes com insuficiencia renal (mediana 40 meses) e sem insuficiencia renal (mediana 83 meses), p = 0,004. No entanto, num modelo multivariavel de Cox, a insuficiência renal nao influencia a sobrevivencia global quando ajustada para o estadiamento International Staging System e a existencia de citogenetica de alto risco. Concluimos que a insuficiencia renal nao e factor prognostico em doentes com mieloma multiplo tratados em primeira linha com novos agentes.
Palavras-chave: Bortezomib; insuficiencia renal; mieloma multiplo; talidomida; transplante autologo de medula.
INTRODUCTION
Multiple myeloma (MM) is a haematological cancer resulting from proliferation of clonalplasma-cells and characterized by the presence of a clonal immunoglobulin in blood and/or urine, with organ dysfunction1.
One of its most common features is renal impairment (RI)2, which can be present at diagnosis in 15 to 40% of patients.
Renal impairment was included in the Durie-Salmon staging system3 and the International Staging System, the most recent prognostic model, also includes RI as a prognostic factor, as β2-microglobulin reflects both the tumour burden and the renal function4.
However, other factors, such as cytogenetic profiling, have been accepted as powerful predictors of survival and are not in staging systems yet5-7.
The new therapeutic agents, bortezomib and thalidomide, have been proven to overcome the adverse prognostic impact of RI, including in a prospective, randomized phase III trial8-12.
We intended to study the impact of RI defined by the Cockcroft-Gault equation, which is more accurate than serum creatinine, as used in the HOVON trial8.
Renal impairment is still an important issue in MM patients and many clinicians believe these patients unfit to receive the best treatment available. Eleftherakis- Papapiakovou et al proved that RI was not an adverse prognostic factor, nevertheless, only 10% of the patients received autologous stem-cell transplant (ASCT)13. Clarifying the prognostic impact of RI in MM patients is, thus, very important when making clinical and therapeutic decisions.
SUBJECTS AND METHODS
We retrospectively analysed patients with MM, treated between July 2004 and December 2012, who have received two cycles of bortezomibor thalidomide-based regimens, and in whom cytogenetic study was performed. We included patients that were treated with high-dose regimens followed by ASCT.
The cytogenetic study was performed using the fluorescence in situ hybridization (FISH) technique. High-risk cytogenetics was defined as having at least of one of the following: translocation4,11, translocation11,14, TP53 gene deletion or gain of chromosome 1. Creatinine clearance was calculated with the Cockcroft-Gault equation and we considered renal impairment (RI) glomerular filtration rate (GFR) below 30mL/min.
The characteristics between the patients with and without RI were compared using the Chi-squared test.
Overall survival (OS) (time from diagnosis to death) and progression free-survival (PFS) (time from diagnosis to death, progression or relapse) were estimated by the Kaplan-Meier method. Survival curves were compared using the log-rank test. The prognostic ability was tested in a univariate analysis and significant prognostic factors were included in a multivariate model. Hazard ratios (HR) and corresponding 95% confidence intervals (95% CI) were determined.
RESULTS
Patient characteristics
We treated 234 patients with MM, but only patients with cytogenetic study were analysed: 174 were considered. Baseline patient characteristics are presented in Table I.
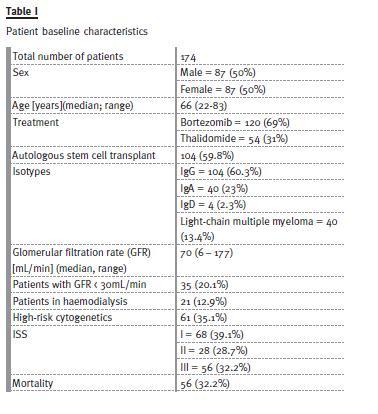
Median follow-up was 30 months (1-101). The proportion of patients in ISS stages was very similar (39.1% in ISS I, 28.7% in ISS 2 and 32.2% in ISS 3). Most patients were treated with bortezomib (69% vs. 31% of patients treated with thalidomide regimens).
ASCT in first-line was performed in 59.8% of patients following a regimen with bortezomib or thalidomide.
The number of patients included with high-risk cytogenetics was 35.1%; 20.1% of patients had GFR under 30mL/min and 12.9% were haemodialysisdependent when treatment began.
The characteristics of patients with RI are summarized in Table II. Median age was 66 years, and 57.1% were treated with bortezomib-based regimens and 42.9% with thalidomide regimens. More than half (51.4%) subsequently underwent ASCT.
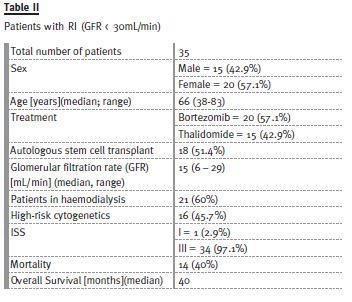
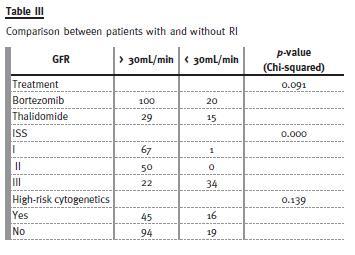
Table III shows the multivariate model for overall survival risk stratification for patients with and without RI. Only ISS significantly differed (with more patients with ISS III in the RI group). There was no difference in treatment regimen or in number of patients with high-risk cytogenetics between the two groups.
Overall survival (OS) and univariate analysis
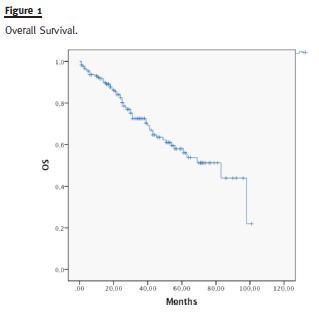
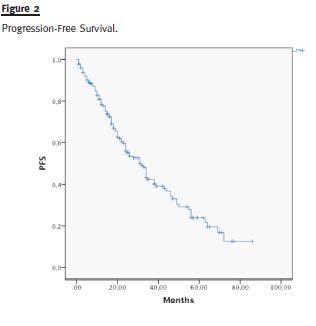
Median OS of the entire group of patients was 83 months (Fig. 1) and median PFS was 42 months (Fig. 2).
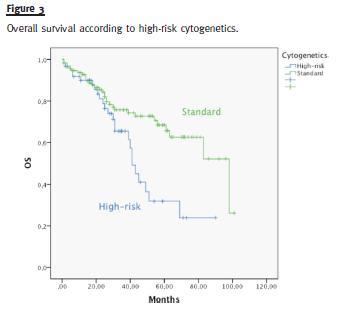
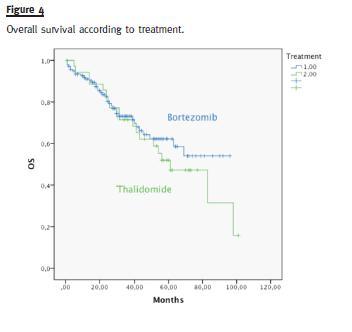
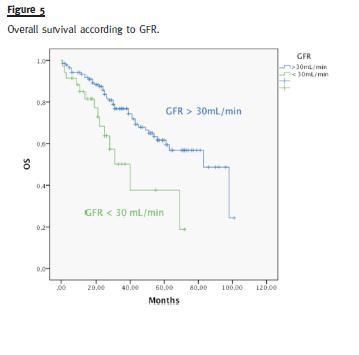
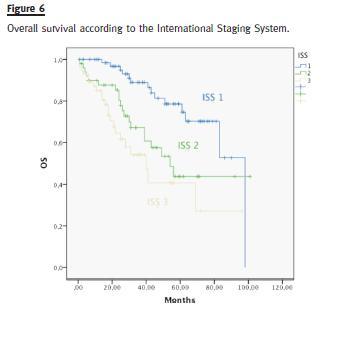
Median OS was different in patients with or without high-risk cytogenetics (41 vs. 98 months, p = 0.006) (Fig. 3). There was no difference in the median OS of patients treated with bortezomib or thalidomide (83 months vs. median not reached, p = 0.593) (Fig. 4). Median OS was significantly superior in patients presenting a GFR above 30mL/min (83 months) than in patients with a GFR < 30mL/min (40 months), p = 0.004 (Fig. 5). Median OS according to ISS was 98, 54 and 40 months to ISS I, II and III. Median OS of ISS I and ISS II were significantly different (p = 0.006), as well as ISS I and ISS III (p = 0.001), but there was no difference between the median OS of ISS II and III (p = 0.174) (Fig. 6).
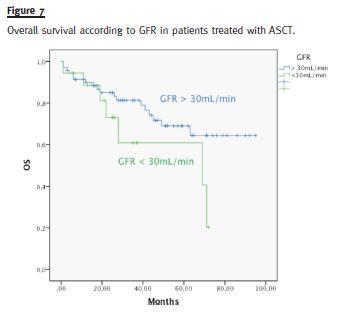
In the subgroup of patients treated with ASCT, median OS of patients with GFR above 30mL/min (median not reached) was not different from patients with GFR below 30mL/min (median 69 months), p = 0. 114 (Fig. 7)
Multivariate analysis
We analysed ISS, GFR and high-risk cytogenetics in a multivariate risk stratification model, as they proved their prognostic ability in a univariate model (Table IV).
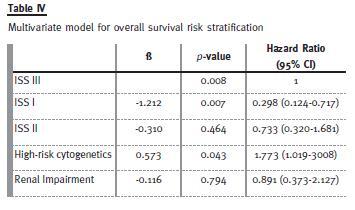
Using ISS 3 as the reference, we observed that ISS I reduced the mortality risk and that high-risk cytogenetics was an adverse risk factor. The RI had not impact OS in this model.
DISCUSSION
The rate of RI was (20.1%) in accordance to the literature, and 12.9% of patients were haemodialysisdependent at presentation. This highlights the importance of the cooperation between nephrologists and haematologists in this subset of patients, in whomis fundamental to try to revert the RI.
Along 8 years (2004-2012), the patients have been treated with different therapeutic regimens as the new therapeutic agents (IMiDs and bortezomib) have become increasingly incorporated into standard firstline regimens for treatment of elderly patients or those eligible for high-dose therapy. The first bortezomib based regimen administrated only occurred by 2009 and became our standard upfront therapy for MM patients since then.
More than half of the patients were treated with ASCT, which clearly overcomes other retrospective studies13.
Despite these pitfalls, the study has a significant number of patients14 in whom cytogenetic analysis was performed, and uses the Cockcroft-Gault equation to estimate the RI, avoiding the mistakes that can be induced by using serum creatinine.
Although not ideal (RIFLE or AKIN should eventually be used, as GFR formulas are only validated in chronic renal disease and not in acute kidney injury12), its superiority against serum creatinine is unquestionable.
The subset of patients with RI had higher ISS (97.1% of patients on stage III) than patients without RI (19.6% of patients on stage III). The majority of patients with RI received bortezomib-based regimens following the recommendations of the International Myeloma Working Group (IMWG). This group shows some reluctance in treating RI patients with thalidomide-based regimens and suggests bortezomib-based regimens16. Interestingly, there was no difference in the OS of patients treated with thalidomide and bortezomib in a univariate analysis.
Univariate analysis showed that RI (GFR < 30mL/min), ISS and high-risk cytogenetics had prognostic ability. Nevertheless, in a multivariate risk stratification model with these variables, RI had not impact OS. This means that despite the difference between the OS of patients with and without RI (40 months versus 83 months), RI should not be analysed isolated.
The ISS, as it includes β2-microglobulin – which measures both RI and the tumoural load – proved to be more a powerful prognostic factor.
The fact that all patients with RI are in the ISS III group explains the difference of OS between patients with and without RI.
Our results are in agreement with other published papers, as the HOVON-65/GMMG-HD4 trial8 that states that MM patients treated with bortezomib overcome the adverse prognosis of RI. The VISTA trial15 also states that a regimen with bortezomib is well tolerated in MM patients with RI and that this complication can be reversed. The data with thalidomide is more limited16, but our results do not appear to be disappointing.
We are aware of the methodological limitations of our work, and in the future we intend to perform a prospective study with the same purpose in patients with MM and RI, applying different RI criteria (RIFLE, if possible) and the renal response criteria, proposed by the IMWG16. However, our study added more information to the RI issue in multiple myeloma. In the era of new therapeutic agents, it is expected that when nephrologists meet a newly diagnosed MM patient with RI, they will refer it, as soon as possible, to a clinical haemato-oncologist so that the treatment could promptly start16,7. The approach of a newly MM patient with RI should be, from now on, ensured by both specialities. Our retrospective analysis demonstrates that when MM patients are treated with new agents, RI at presentation is not an adverse prognostic factor.
References
1. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046-1060. [ Links ]
2. Alexanian R, Barlogie B, Dixon D. Renal failure in multiple myeloma. Pathogenesis and prognostic implications. Arch Intern Med 1990;150(8):1693-1695. [ Links ]
3. Durie BG, Salmon S E. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer 1975;36(3):842-854. [ Links ]
4. Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol 2005;23(15):3412-3420. [ Links ]
5. Avet-Loiseau H, Attal M, Moreau P, et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. Blood. 2007;109(8):3489-3495. [ Links ]
6. Keats JJ, Reiman T, Maxwell CA, et al. In multiple myeloma, t(4;14)(p16;q32) is an adverse prognostic factor irrespective of FGFR3 expression. Blood 2003;101(4):1520-1529. [ Links ]
7. Chang H, Qi C, Yi QL, Reece D, Stewart AK. P53 gene deletion detected by fluorescence in situ hybridization is an adverse prognostic factor for patients with multiple myeloma following autologous stem cell transplantation. Blood 2005;105(1):358–360. [ Links ]
8. Scheid C, Sonneveld P, Schmidt-Wolf IG, et al. Bortezomib before and after autologous stem cell transplantation overcomes the negative prognostic impact of renal impairment in newly diagnosed multiple myeloma: a subgroup analysis from the HOVON-65/GMMG-HD4 trial. Haematologica 2014;99(1):148-154. [ Links ]
9. Badros A, Barlogie B, Siegel E, et al. Results of autologous stem cell transplant in multiple myeloma patients with renal failure. Br J Haematol 2001;114(4):822-829. [ Links ]
10. San Miguel JF, Lahuerta JJ, Garcia-Sanz R, et al. Are myeloma patients with renal failure candidates for autologous stem cell transplantation? Hematol J 2000;1(1):28-36. [ Links ]
11. Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008;111(5):2516-2520. [ Links ]
12. Kastritis E, Zervas K, Symeonidis A, et al. Improved survival of patients with multiple myeloma after the introduction of novel agents and the applicability of the International Staging System (ISS): an analysis of the Greek Myeloma Study Group (GMSG). Leukemia 2009;23(6):1152-1157. [ Links ]
13. Eleftherakis-Papapiakovou E, Kastritis E, Roussou M, et al. Renal impairment is not an independent adverse prognostic factor in patients with multiple myeloma treated upfront with novel agent-based regimens. Leuk Lymphoma 2011;52(12):2299-2303. [ Links ]
14. Kyle RA, Rajkumar SV. Multiple myeloma. Blood 2008;111(6):2962-2972. [ Links ] [ Links ]
16. Chanan-Khan AA, San Miguel JF, Jagannath S, Ludwig H, Dimopoulos MA. Novel therapeutic agents for the management of patients with multiple myeloma and renal impairment. Clin Cancer Res 2012;18(8): 2145–2163. [ Links ]
Dr. Manuel Neves
Department of Haemathology and Bone Marrow Transplantation
Centro Hospitalar Lisboa Norte – Hospital Santa Maria
Avenida Professor Egas Moniz
1600-190 Lisboa, Portugal
E-mail: mleaoneves@gmail.com
Conflict of interest statement: GE is member of the advisory board for Janssen and Celgene; all the others authors have no conflict of interest to declare.
Received for publication: 03/07/2014
Accepted in revised form: 04/09/2014














