Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.28 no.4 Lisboa dez. 2014
ORIGINAL ARTICLE
Long nocturnal dialysis: A single-centre experience
Diálise longa noturna: Experiência de um centro
David Navarro1,2, Ana Carina Ferreira1,2,3, Carina Gonçalves1, Cristina Jorge1,2, Célia Gil1,2, Inês Aires1,2,3, Patrícia Matias1,2, Marco Mendes1,2, Ana Azevedo1,2, Fernanda Gomes1,2, Aníbal Ferreira1,2,3
1 Nephrocare Vila Franca de Xira. Lisbon, Portugal
2 NIDAN – Núcleo de Investigação e Desenvolvimento na Área Nefrológica
3 Faculdade de Ciências Médicas da Universidade Nova de Lisboa. Lisbon, Portugal
ABSTRACT
The institution of intensified dialysis regimens (as long treatment time, with reduced ultrafiltration per hour) has been associated with decreased morbidity and mortality in patients with end-stage chronic kidney disease. The performance of the haemodialysis session during the night interval emerged as logical, since it is an idle period, and has been associated with better small molecule dialysis, better blood pressure control, reduced medication requirements and improved quality of life. Recently, our centre initiated a long nocturnal dialysis programme and a prospective observational analysis was designed to evaluate the results of this approach. Mean values of clinical and laboratory variables were compared in 2 consecutive semesters: prior and after transition from haemodiafiltration to long nocturnal dialysis. After 6 months of switching, there was an increase in dialysis efficiency (reduction in pre-dialysis urea (129.74 ± 28.7 vs. 114.53 ± 23.94 mg/dl, p = 0.01) and an increase in Kt/V (1.75 ± 0.37 vs. 2.09 ± 0.39, p = 0.005)), improved hyperphosphatemia control (5.05 ± 0.9 vs. 4.23 ± 0.93 mg/dl; p = 0.01) and anaemia control, with a significant reduction in the use of darbepoetin alfa (38.5 ± 24.18 vs. 30.83 ± 22.54 μg/week; p = 0.04) and of intravenous iron (189.33 ± 117 vs. 116 ± 67 mg/month; p = 0.04) and a much better correction of overhydration (evaluated by the BCM-Body composition monitor: 10.2% ± 8.63 vs. 4.6% ± 7.2; p = 0.01), reflecting the patients overall better nutritional status. These excellent results were amplified by the patients perception of improvement in their quality of life. Our findings are consistent with the studies that favour long nocturnal dialysis over conventional regimens, but randomized controlled trials are needed to validate these findings.
Key-words: Anaemia; blood pressure; mineral and bone disease; nocturnal dialysis; outcome; quality of life.
RESUMO
Os esquemas de diálise longa, com taxas de ultrafiltração/hora mais baixas, têm sido associados a menor mortalidade, na maioria dos estudos. Neste contexto, a diálise longa noturna surgiu como uma modalidade de diálise alternativa e atrativa. A diálise longa noturna tem sido associada a maior eficácia dialítica, melhor controlo tensional, com diminuição das necessidades medicamentosas e melhor qualidade de vida. O nosso centro iniciou recentemente um programa de diálise longa noturna cujos resultados nos propusemos analisar num estudo prospetivo e observacional. Com este objectivo, avaliámos a evolução de variáveis clínicas e analíticas em 2 semestres consecutivos, antes e após a transição de hemodiafiltração para diálise longa noturna. Seis meses após a transição, verificámos uma melhoria da eficiência dialítica (redução de ureia pré-diálise (129.74 ± 28.7 vs. 114.53±23.94 mg/dl, p=0.01) e subida de Kt/V (1.75 vs. 2.09 ± 0.39, p= 0.005)), do controlo da hiperfosfatemia (5.05 ± 0.9 vs. 4.23 ± 0.93 mg/dl; p = 0.01) e da anemia, com significativa redução do consumo de darbepoetina alfa (38.5 ± 24.18 vs. 30.83 ± 22.54 μg/semana; p = 0.04) e de ferro endovenoso (189.33 ± 117 vs. 116 ± 67 mg/mês; p = 0.04), bem como uma excelente correção do estado de híper-hidratação (avaliado pelo monitor de BCM-Body composition monitor: 10.2% ± 8.63 vs. 4.6% ± 7.2; p = 0.01), reflexo do melhor estado nutricional dos doentes. A estes benefícios acresceuse a perceção de melhoria da qualidade de vida dos doentes. O nosso estudo vem juntar-se à lista de trabalhos cujos resultados favorecem a diálise longa noturna em relação à hemodiálise convencional, mas são necessários estudos randomizados para validar estes resultados.
Palavras-chave: Anemia; diálise nocturna; doença óssea; pressão arterial; qualidade de vida; resultados.
INTRODUCTION
The institution of intensified dialysis regimens (long treatment time, with reduced ultrafiltration per hour) has been associated with decreased morbidity and mortality in patients with end-stage chronic kidney disease (CKD)1,2. The option for a longer dialysis session is based on the principle that it enables a more physiological process, allowing for the removal of larger volume of fluids and toxins. It also results in fewer side-effects, including hypotension and/or cramps. However, the most important benefit is the improved survival of patients, initially evidenced by the Tassin group3 and, more recently, by other investigators4,5.
In this context, the performance of the haemodialysis session during the night emerged as logical, since it is an idle period. In addition to the direct benefits stated previously, the indirect advantage of being able to maintain daily activities, with much less restrictions than with conventional daytime sessions, are far from negligible.
Since the late 1960s the Tassin (France) group has adopted long nocturnal dialysis (LND) – 8 hours, 3 times a week – and is now the largest centre of expertise in nocturnal dialysis worldwide. This incentre nocturnal dialysis regimen offers the advantages of medical staff supervision, while allowing patients to maintain their daily activities. In Portugal, the LND was introduced in 1983, at the Clínica de Doenças Renais (Lisbon). Since then, the experience has been replicated in several Portuguese centres.
In April 2013, it was the turn of the Nephrocare – Vila Franca de Xira haemodialysis centre to implement an LND programme, creating the opportunity for prospective evaluation. The aim of this study was to find clinical advantages in anaemia, bone metabolism and dialysis dose, from the switch to LND.
SUBJECTS AND METHODS
Study Design
This was a prospective, single-centre study, of a cohort of prevalent haemodialysis patients, switched from daytime on-line haemodiafiltration (HDF) postdilution (mean time: 240 minutes/session) to LND (mean time: 395 minutes/session). The patients were followed for, at least, 6 months before and 6 months after the switch. Clinical and laboratory data were assessed monthly, and compared as median values pre-LND and post-LND.
Patients
We evaluated 10 patients, six males, and four females, with mean age of 41.2 ± 7.21 years, and mean dialysis time of 61 ± 38.4 months. Two patients were diabetic, four had arterial hypertension, and one patient had hepatitis C infection.
All patients underwent dialysis with Fresenius® equipment and ultrapure water (endotoxine-free, evaluated monthly with chromogenic kinetic LAL assay). Nine patients used high-flux synthetic membranes, while one required the use of a cellulose triacetate membrane. Vascular access was as follows: 6 arteriovenous fistulas and 4 arteriovenous grafts. All vascular access were subjected to ropeladder cannulation technique. The average blood flow rate was 310 ml/min, with an autoflow dialysate rate of 1.0. Systolic and diastolic blood pressure (BP) was measured in all dialysis sessions during the study period, and also kt/v, using OCM module. Normohydration/overhydration (OH) status was evaluated by monthly BCM-bioimpedance spectroscopy. Hypotensive drugs were evaluated at the beginning (6 months before LND) and at the end (6 months after LND) of the study, using the antihypertensive drug index (ADI) – the ratio of the dose of the drug used by its maximum. Erythropoietin stimulating agents (ESA), iron and phosphate binders therapeutic doses were also collected monthly.
The Kidney Disease Quality of Life Short Form (KDQL-SF) was applied to assess the patients quality of life, after the switch to LND.
Biochemical analysis
Serum levels of haemoglobin, pre-dialysis urea, potassium, phosphorous, calcium, and C-reactive protein were measured monthly, while ferritin, intact parathyroid hormone (PTH), and albumin were obtained every 3 months.
Biochemical analysis including haemoglobin, calcium, phosphorus, ferritin, and albumin was performed using standard methods. The PTH was measured by immunochemiluminescence using a second-generation assay and the normal range is 10 to 65 pg/ml.
Echocardiographic evaluation
Our patients were annually evaluated by echocardiogram, M mode. Left ventricular mass index (LVMI) was calculated using Devereux formula and indexed to body surface area. The presence of left ventricular hypertrophy (LVH) was defined based on a LVMI > 125 g/m2 for both genders. Ejection fraction was measured using Simpsons modified rule.
Statistical analysis
Data are presented as frequencies for categorical variables, and mean ± SD values for continuous normally distributed variables. Because of the sample size (10 patients), comparisons between HDF period vs. LND period was made using non-parametric test Wilcoxon for paired samples.
All tests were performed using STATA software version 13, and a p-value < 0.05 was considered statistically significant.
RESULTS
Clinical and demographic characteristics of patients are listed in Table I. The average results before and after the transition to LND are summarized in Table II.
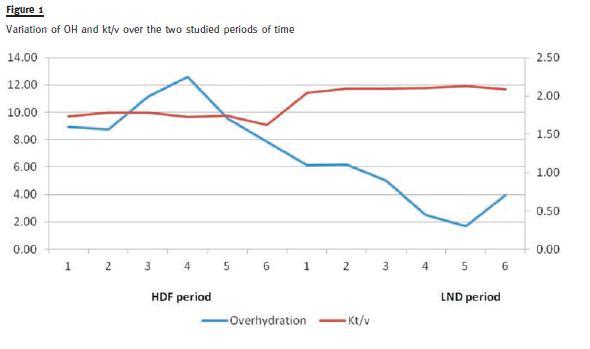
With more intense dialysis, we observed a reduced pre-dialysis urea (129.74 ± 28.7 vs. 114.53 ± 23.9 mg/dl, p < 0.001), and increased average Kt/V (1.75 ± 0.4 vs. 2.09 ± 0.4, p = 0.005) – see Fig. 1.
Average systolic BP remained stable 6 months after the switch, with a concomitant progressive decrease in antihypertensive therapy (quantified by the ADI): 3.69 in the month before the transition vs. 2.17 at 6 months after transition, p = 0.15. Echocardiography findings indicating left ventricular mass and ejection fraction remained unchanged 9 months after transition (6-12 months).
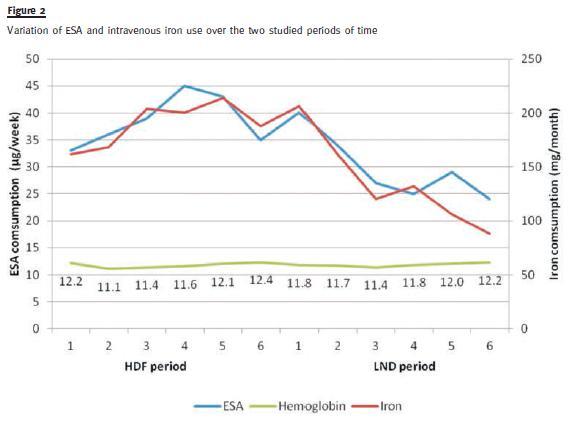
We verified a correction in overhydration (evaluated by the BCM-Body composition monitor: 10.2%± 8.6 vs. 4.6% ± 7.2; p = 0.01), while our patients weight remained stable There was a significant decline in ESA use (darbepoetin doses were reduced from 38.5 ± 24.2 to 30.83 ± 22.5 <μg/week; p = 0.04) and in intravenous iron use (189.33 ± 12 vs. 116 ± 7 mg/month; p = 0.04), with comparable hemoglobin levels, as shown in Fig. 2. Ferritin, C-reactive protein and albumin levels did not differ between the two time periods.
A reduction in the mean phosphorous level (5.05 ± 0.9 vs, 4.23 ± 0.9 mg/dl; p = 0.01), and a concomitant increase in the mean calcium level (8.59 ± 0.3 vs. 9.1 ± 0.3 mg/dl; p = 0.005) were observed. An upward trend was noted in PTH levels, although not statistically significant (328.1 ± 161.5 vs. 385.9 ± 241.5 pg/ml; p = 0.38). Phosphate binders were used by nine patients prior to transition, with six of them either reducing or discontinuing its use.
We did not witness an increase in vascular access complications (3 vs. 1).
Six months after the transition, we applied the Kidney Disease Quality of Life Short Form (KDQL-SF) to assess our patients quality of life, and observed a statistically significant improvement in the patients perception of their health, sensation of breathlessness, anorexia, perception of fatigue and the degree of satisfaction with the amount of time spent with family and friends.
DISCUSSION
Overall, and in a short period of time, our results finds attractive effects of LND. Let us appreciate them separately.
LND and Clearance of solutes
Several studies have shown that prolonged dialysis schemes, including LND, are clearly superior with regard to the clearance of solutes when compared with conventional haemodialysis or HDF. This is reflected by lower urea6, phosphate7 and beta-2microglobulin8 serum levels and by an increase in the value of Kt/V9.
Similar changes were observed in the study conducted in our centre. While the HEMO trial10 reported no significant impact of the Kt/V dose on survival after 5 years of follow-up, replacing kidney function is not restricted to the clearance of small molecules. Other factors, such as nutrition, extracellular volume and blood pressure need to be optimized to achieve optimal dialysis, and they are easier to attain with a longer or more frequent dialysis sessions. While the ideal dose of dialysis is hard to determine, the long session thrice weekly is at least thought-provoking.
LND and Cardiovascular effects
Regarding cardiovascular effects, LND has shown marked benefits, with improved BP control being reported across most studies11. The Tassin group documented survival improvement in their patients, verifying that this is predominantly due to a lower cardiovascular mortality5. In turn, these effects were more pronounced in the subgroup of patients with better BP control. This aspect is reflected directly with lower pre-and post-dialysis BP9, as well as indirectly by a reduced need for antihypertensive medications11, probably reflecting the greater facility in reaching the normohydrated weight with longer dialysis sessions. The benefits of LND and better BP control are also demonstrated by a regression of left ventricular mass12 and improved left ventricular ejection fraction13. Some argue that improved BP control is, at least partially, due to a better vascular function, through its total peripheral resistance lowering effect14. Of mention, coronary calcification rate in LND was analysed and, although further studies are needed to confirm these observations, it appears to be significantly lower than that observed in conventional regimens15.
These findings are probably related to the great amelioration in calcium/phosphate metabolism described below.
In our patients, it was possible to steadily cutback in antihypertensive therapy while maintaining their average systolic BP. While the echocardiography findings regarding left ventricular mass and ejection fraction remained unchanged, it must be mentioned that echocardiography evaluation was not performed, neither optimized, to document these changes.
LND and Anaemia & Nutrition
Anaemia control is another frequently stated advantage of nocturnal dialysis16. However, negative results have also been published17. In our group, we observed a substantial decline in ESA and in intravenous iron use, with other parameters, as haemoglobin, ferritin and C-reactive protein levels, remaining unchanged.
Despite these results being in line with previous studies, we cannot ignore the possible effect of the correction in overhydration status that we also verified.
This result, in association with the fact that our patients weight remained stable, reflects their overall better nutritional status18. More intensive dialysis regimens have been associated with better nutrition, more liberated dietary intake, and with higher protein intake (frequently encouraged, since the loss of aminoacids is higher in prolonged dialysis regimens19).
Albumin levels in these patients usually remain steady20, and the same happened in our group of patients. Furthermore, dyslipidemia may also improve in LND, as it is associated with uraemia in a syndrome characterized by an elevation of triglyceride rich lipoproteins, a reduction in high density lipoprotein (HDL) levels, and a higher fraction of atherogenic, small dense low density lipoprotein (LDL)21. We did not study this potential effect of LND.
LND and CKD-MBD
Patients under LND usually have better mineral bone disease control, given the central role of phosphate levels in the prevention and treatment of secondary hyperparathyroidism. Because of its molecular weight, phosphate removal in dialysis is dependent on the sessions duration22, being naturally higher in this group of patients – an effect that we also verified, with a reduction in the mean phosphorous level, and an increase in the mean calcium level. The later can be justified, at least partly, by the increase in PTH serum levels we observed and the reduction of precipitation of the calcium phosphate complexes.
It is not clear what the effect of LND on PTH levels is due to many contradicting studies9,23,24. Some justify a decrease in PTH levels with the improved phosphate control, while others explain its increase with the negative calcium balance.
Importantly, despite the more liberal dietary intake previously stated, a reduction in the use of phosphorus binders is a common finding in LND23. We also observed this trend in our group of patients, with six out of nine either reducing or discontinuing its use.
LND and Quality of Life
Patients under haemodialysis have low quality of life due, in part, to the amount of time spent with dialysis treatment during the day. This has a huge impact in their daily personal, social, and professional activities, and a high rate of unemployment is the norm. Besides the physiological benefits of longer dialysis, the fact that nocturnal dialysis is performed during an idle period grants to patients the possibility to restore their daily activity. These indirect benefits are hard to quantify, but certainly aids the patients well-being and overall quality of life. This has been documented previously25, with patients appearing to prefer nocturnal dialysis. Reduction in pill burden observed in these studies is also important for the patient, since high pill burden results in high rates of medication non-adherence26. One domain where benefits would not be expected is sleep quality, but since disturbances are minimal, sleep architecture actually appears to improve. This finding may be related with the improved uraemic toxins clearance discussed previously27. Still, some patients might find it hard to get comfortable sleep positions, for fear of alarm generation or needle displacement.
As mentioned above, a statistically significant improvement in the patients perception of their quality of life was observed. Additionally, three of our 10 patients are currently employed, and refer that the transition has greatly facilitated their daily activities.
Shortcomings
There are also a number of potential disadvantages to LND that have to be considered. Some patients find it hard to sleep away from home three times a week. Patients also have a more limited access to multidisciplinary staff, such as vascular access centre, dietician or social assistant. It has been verified that more frequent dialysis regimens are associated with more pronounced residual renal function decline, which in turn is correlated with lower survival among incident dialysis patients28.
Another concern is the fact that more prolonged treatments are associated with higher rate of vascular access complications29. Nevertheless, these events seem to be related to higher frequency of treatment (and cannulation) rather than with its duration30. Studies addressing this particular issue are needed.
Still, one must be cautious when analysing LND studies, since patient selection biases with younger and healthier patients choosing this modality are frequent and hard to avoid. In our study, patients were observed before and after the switch to LND, and each patient was its own case and control, thereby removing the problem of selection bias.
CONCLUSION
While this review is somewhat compartmentalized, it is clear that most benefits are interlinked: benefits of intensified clearance result in better nutrition, which in turn has effects on anaemia and CKD-mineral and bone disorders. After 6 months of switching from a high efficient dialysis (on line HDF) to LND, even in a study with a small sample size, there was a substantial increase in dialysis efficiency, control of hyperphosphatemia and anaemia (with a significant reduction in the use of ESA and iron), and a much better correction of overhydration. These excellent results were amplified by the patients perception of improvement in their quality of life.
The benefits must, therefore, be balanced with the shortcomings, and while individual studies have generally been supportive of LND over conventional HD, the magnitude of improvement in specific parameters has varied markedly between studies, and randomized controlled trials are needed to validate these findings.
References
1. Saran R, Bragg-Gresham JL, Levin NW, et al. Longer treatment time and slower ultrafiltration in hemodialysis: associations with reduced mortality in the DOPPS. Kidney Int 2006;69(7):1222-1228. [ Links ]
2. Nesrallah GE, Lindsay RM, Cuerden MS, et al. Intensive hemodialysis associates with improved survival compared with conventional hemodialysis. J Am Soc Nephrol 2012;23(4):696-705. [ Links ]
3. Charra B, Chazot C, Jean G, et al. Long 3 x 8 hr dialysis: a three-decade summary. J Nephrol 2003;16 (Suppl 7):S64-S69. [ Links ]
4. Lacson E Jr, Xu J, Suri RS, et al. Survival with three-times weekly in-center nocturnal versus conventional hemodialysis. J Am Soc Nephrol 2012;23(4):687-695. [ Links ]
5. Charra B, Jean G, Chazot C, et al. Intensive dialysis and blood pressure control: A review. Hemodial Int 2004;8(1):51-60. [ Links ]
6. Powell JR, Oluwaseun O, Woo YM, et al. Ten years experience of in-center thrice weekly long overnight hemodialysis. Clin J Am Soc Nephrol 2009;4(6):1097-1101. [ Links ]
7. Pierratos A, Ouwendyk M. Nocturnal hemodialysis: five years later. Semin Dial 1999;12(6):419-423. [ Links ]
8. Raj DS, Ouwendyk M, Francoeur R, Pierratos A. Beta2-microglobulin kinetics in nocturnal haemodialysis. Nephrol Dial Transplant 2000;15(1):58-64. [ Links ]
9. David S, Kümpers P, Eisenbach GM, Haller H, Kielstein JT. Prospective evaluation of an in-centre conversion from conventional haemodialysis to an intensified nocturnal strategy. Nephrol Dial Transplant 2009;24(7):2232-2240. [ Links ]
10. Eknoyan G, Beck GJ, Cheung AK, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 2002;347(25):2010-2019. [ Links ]
11. Walsh M, Culleton B, Tonelli M, Manns B. A systematic review of the effect of nocturnal hemodialysis on blood pressure, left ventricular hypertrophy, anemia, mineral metabolism, and health-related quality of life. Kidney Int 2005;67(4):1500-1508. [ Links ]
12. Culleton BF, Walsh M, Klarenbach S, et al. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. JAMA 2007;298(11):1291-1299. [ Links ]
13. Chan C, Floras JS, Miller JA, Pierratos A. Improvement in ejection fraction by nocturnal haemodialysis in end-stage renal failure patients with coexisting heart failure. Nephrol Dial Transplant 2002;17(8):1518-1521. [ Links ]
14. Chan CT, Harvey PJ, Picton P, Pierratos A, Miller JA, Floras JS. Short-term blood pressure, noradrenergic, and vascular effects of nocturnal home hemodialysis. Hypertension 2003;42(5):925-931. [ Links ]
15. Yuen D, Pierratos A, Richardson RM, Chan CT. The natural history of coronary calcification progression in a cohort of nocturnal haemodialysis patients. Nephrol Dial Transplant 2006;21(5):1407-1412. [ Links ]
16. Schwartz DI, Pierratos A, Richardson RM, Fenton SS, Chan CT. Impact of nocturnal home hemodialysis on anemia management in patients with end-stage renal disease. Clin Nephrol 2005;63(3):202-208. [ Links ]
17. Rocco MV, Lockridge RS Jr, Beck GJ, et al. The effects of frequent nocturnal home hemodialysis: the Frequent Hemodialysis Network Nocturnal Trial. Kidney Int 2011;80(10):1080-1091. [ Links ]
18. Garagarza C, João-Matias P, Sousa-Guerreiro C, et al. Nutritional status and overhydration: can bioimpedance spectroscopy be useful in haemodialysis patients? Nefrologia 2013;33(5):667-674. [ Links ]
19. Ikizler TA, Flakoll PJ, Parker RA, Hakim RM. Amino acid and albumin losses during hemodialysis. Kidney Int 1994;46(3):830-837. [ Links ]
20. Kaysen GA, Greene T, Larive B, et al. The effect of frequent hemodialysis on nutrition and body composition: Frequent Hemodialysis Network Trial. Kidney Int 2012;82(1):90-99. [ Links ]
21. Bugeja AL, Chan CT. Improvement in lipid profile by nocturnal hemodialysis in patients with end-stage renal disease. ASAIO J 2004;50(4):328-331. [ Links ]
22. Jakubovic BD, Yan AT, Wald R. In-center nocturnal hemodialysis. Semin Dial 2014;27(2):179-187. [ Links ]
23. Williams AW, OSullivan DA, McCarthy JT. Slow nocturnal and short daily hemodialysis: A comparison. Semin Dial 1999;12(6):431-439. [ Links ]
24. Haag-Weber M. Treatment options to intensify hemodialysis. Kidney Blood Press Res 2003;26(2):90-95. [ Links ]
25. Van Eps CL, Jeffries JK, Johnson DW, et al. Quality of life and alternate nightly nocturnal home hemodialysis. Hemodial Int 2010;14(1):29-38. [ Links ]
26. Karamanidou C, Clatworthy J, Weinman J, Horne R. A systematic review of the prevalence and determinants of nonadherence to phosphate binding medication in patients with end-stage renal disease. BMC Nephrol 2008;9:2. [ Links ]
27. Perl J, Unruh ML, Chan CT. Sleep disorders in end-stage renal disease: Markers of inadequate dialysis? Kidney Int 2006;70(10):1687-1693. [ Links ]
28. Diaz-Buxo JA, White SA, Himmele R. Frequent hemodialysis: A critical review. Semin Dial 2013;26(5):578-589. [ Links ]
29. Suri RS, Larive B, Sherer S, et al. Risk of vascular access complications with frequent hemodialysis. J Am Soc Nephrol 2013;24(3):498-505. [ Links ]
30. Jun M, Jardine MJ, Gray N, et al. Outcomes of extended-hours hemodialysis performed predominantly at home. Am J Kidney Dis 2013;61(2):247-253. [ Links ]
Dr. David Navarro
Nephrocare Vila Franca de Xira, Dialysis Clinic.
Praça Bartolomeu Dias, Lote 3 R/C
2600-076 Vila Franca de Xira, Portugal
E-mail: davidbnavarro@gmail.com
Conflict of interest statement: The author AF received grants from Abbott, Abbvie, Amgen, Genzyme, OM Pharma and Shire. Member of national and international Advisory Boards for Abbott, Abbvie, Amgen, Fresenius Medical Care, Genzyme OM Pharma and Shire.
Received for publication: 11/07/2014
Accepted in revised form: 20/08/2014














