Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.28 no.3 Lisboa set. 2014
ORIGINAL ARTICLE
Transplant glomerulopathy and post-transplant de novo thrombotic microangiopathy: common features and pathologic mechanisms
Glomerulopatia do transplante e microangiopatia trombótica de novo pós transplante: morfologia e mecanismos patológicos
Rui M Costa1, Eduardo Vazquez -Martul2, Juan Reboredo -Mosquera2, Constantino Rivera3
1 Nephrology Department, CH Trás -os -Montes e Alto Douro. Vila Real, Portugal.
2 Pathology Department, CH Juan Canalejo. A Coruña, Spain.
3 Nephrology Department, CH Juan Canalejo. A Coruña, Spain.
ABSTRACT
Aim: Comparison between transplant glomerulopathy (TG) and de novo thrombotic microangiopathy (TMA) in renal allograft biopsies in a 10 -year retrospective analysis. Results: Out of a total of 627 biopsies, TG (6.2%) was diagnosed at a later stage (6.9 ± 5.9 vs. 3.5 ± 6.5 years, p = 0.01) and presented higher proteinuria (4.0 ± 3.6 vs. 2.3 ± 1.6 gr/24h, p = 0.02), advanced glomerulosclerosis, interstitial fibrosis/tubular atrophy and interstitial plasma cells (30.8% vs. 0%, p = 0.02). De novo TMA (2.2%) was associated to worse graft disfunction (sCr 4.9 ± 1.8 vs. 2.7 ± 0.2mg/dl, p < 0.01), older donors (53 ± 10 vs. 41 ± 17 years, p = 0.04), PRA levels ≥ 25% (16.7% vs. 3.1%, p = 0.07) and previous rejection events (21.4% vs. 5.3%, p = 0.08). Diffuse glomerular lesions were observed in all TMA cases, with capillary congestion (100 vs. 35.1%, p < 0.01), microthrombi (50% vs. 5.4, p = 0.01), schistocytes (42.8% vs. 7.7%, p = 0.01) and mesangiolysis (85.7% vs. 29.7%, p < 0.01). Positive C4d in de novo TMA cases was similar to TG (71.4% vs. 53.8%, p = ns) but presented arteriolar C4d deposition (35.7 % vs. 8.7%, p = 0.042). Donor -specific antibodies detection was equally found (TG: 41.6%; TMA: 57.1%, p = ns), mainly anti–HLA Class II. In ultrastructural analysis only TG cases presented glomerular basement membrane (GBM) multilayering. Graft loss was similar, but de novo MAT cases had worst first year survival (73.3% vs. 97%, p = 0.013). Conclusion: Both pathologies belong within the spectrum of microcirculatory injury: TG results from subclinical lesion, presenting chronic cyclic accommodation, de novo TMA represents severe form of lytic endothelial lesion.
Key-Words: Antibody-mediated rejection; kidney transplant; thrombotic microangiopathy; transplant glomerulopathy.
RESUMO
Objectivo: Comparação retrospectiva entre a glomerulopatia de transplante (GT) e microangiopatia trombótica (MAT) de novo num período de 10 anos de biópsias de enxertos renais. Resultados: No total de 627 biopsias, GT (6,2%) foi diagnosticada mais tardiamente (6.9 ± 5.9 vs. 3.5 ± 6.5 anos, p = 0.01), com maior grau de proteinúria (4.0 ± 3.6 vs. 2.3 ± 1.6 gr/24h, p = 0.02), glomeruloesclerose, fibrose intersticial e atrofia tubular mais severas e presença de plasmócitos intersticiais (30.8% vs. 0%, p = 0.02). Os casos de MAT de novo >(2,2%) apresentavam disfunção do enxerto mais severa (sCr 4.9 ± 1.8 vs. 2.7 ± 0.2mg/dl, p < 0.01) e associação com dadores mais velhos (53 ± 10 vs. 41 ± 17 anos, p = 0.04), níveis de PRA ≥ 25% (16.7% vs. 3.1%, p = 0.07) e episódios prévios de rejeição aguda (21.4% vs. 5.3%, p = 0.08). Lesões glomerulares difusas foram detectadas em todos os casos de MAT, especialmente com congestão capilar (100% vs>. 35.1%, p < 0.01) e predomínio de presença de microtrombos (50% vs>. 5.4 %, p = 0.01), esquizócitos (42.8% vs. 7.7%, p = 0.01) e mesangiólise (85.7% vs. 29.7%, p < 0.01). Positividade para C4D em MAT de novo e TG foi semelhante (71.4% vs. 53.8%, p = ns) mas a deposição arteriolar de C4d foi mais frequente em MAT de novo (35.7 % vs. 8.7%, p = 0.042). A detecção de anticorpos anti -dador foi similar entre os dois grupos (GT: 41.6%; MAT: 57.1%, p = ns) e constituído maioritariamente por anticorpos anti-HLA Classe II. Apenas a GT apresentava multilaminação da membrana basal glomerular na microscopia electrónica. A perda do enxerto foi semelhante entre as duas patologias mas MAT de novo apresentou pior sobrevida renal ao primeiro ano (73.3% vs>. 97%, p = 0.013). Conclusão: GT e MAT de novo são expressões histológicas que se incluem num espectro de lesão da microcirculação: a GT resulta de lesão subclínica, apresentando acomodação crónica cíclica e a MAT de novo expressa uma forma severa de lise endotelial.
Palavras-chave: Glomerulopatia do transplante; microangiopatia trombótica, rejeição mediada por anticorpos; transplante renal.
INTRODUCTION
Transplant glomerulopathy (TG) has been described as a late manifestation of allograft injury, with 5-year incidence reaching 20% and is expressed with proteinuria (usually in nephrotic range), hypertension and declining function with consequent poor graft outcome1. Clinical presentation is usually late, occurring years after transplantation, and advanced glomeruloesclerosis, intersticial fibrosis, tubular atrophy and vasculopathy is frequently found. It is believed to result from chronic microcirculation injury in close relation with antibody-mediated rejection (AMR) due to the frequent association to C4d deposition and greater incidence in patients with previous humoral rejection episodes and donor specific antibodies (DSA) detection2.
However, a substantial number of TG cases have no C4d deposits or DSA in circulation, where non-alloantibody mediated processes may be involved in a subset of patients. Association between hepatitis C (HCV) membrano-proliferative glomerulonephritis (MNPG) and TG may be evident based on the similarities between histopathological features. The low levels and/or reabsorption of immune complexes because of the immunosuppression, causing negative immunofluorescence, may make it hard to clearly differentiate HCV -associated MNPG and TG since chronic glomerular lesions are similar in these pathologies.
Baid-Agrawal et al3 have shown frequent prevalence of HCV infection in TG cases (36%), and pre -transplant HCV positivity was identified as risk factor for TG development and rapid progression to graft failure.
Another disorder capable of histopathological findings analogous to TG is de novo thrombotic microangiopathy (TMA) in renal allograft. It usually occurs in the early post -transplantation period (first 3 months), but indolent occurrence detected at late stages is also described4. Causes for de novo TMA includes toxicity of calcineurin inhibitors (CNI) and mTor inhibitors, viral infection (HCV and CMV) and ischaemia-reperfusion injury. Humoral rejection is also believed to be an important cause for de novo TMA, due to the positive peritubular capillary (PTC) C4d staining in this cases and overlap of histopathologic features with antibody -mediated rejection (AMR)5.
Association between TG and de novo MAT can be observed in the spectrum of abnormalities: glomerular hypercellularity, endothelial cell swelling with loss of fenestrations, subendothelial widening, glomerular basement membrane (GBM) thickening with duplication and mesangiolysis are present in both pathologies in early phases6. Renal microvascular endothelium injury seems to be responsible for similar morphologic characteristics between TMA and TG: glomerular and PTC injury occurs, suggesting that both diseases represent a pancapilllaritis of the renal allograft.
Rather than a cause, we believe that de novo TMA and TG are included within the same spectrum of pathologic manifestations of endothelial lesion.
This study compares clinical, laboratory and pathologic features in TG and de novo TMA patients in a single centre. Our purpose is to evaluate similarities and differences between both entities, taking into account the microvascular endothelial injury as a pathologic trigger shared by both diseases.
MATERIAL AND METHODS
We performed a retrospective study reviewing all allograft biopsies with C4d staining and histological diagnosis of TG or de novo TMA performed in our unit, between January 2003 and December 2012.
Biopsy was performed based on clinical indications (graft disfunction or proteinuria) and not in a protocol manner. Recurrent TMA (n = 6) cases were excluded.
In repeat biopsies only the first sample was considered.
A systematic histological analysis of the biopsies was carried out on haematoxylin and eosin-stained, periodic acid -Schiff -stained and trichrome – stained slides by two dedicated nephropathologists. All biopsies presented at least 10 glomeruli and two arterioles. Meticulous search was made for changes suggestive of cellular or antibody-mediated rejection according to the Banff 2007 criteria.
Graduation of histological features was not performed because that could lead to an underpowered statistical analysis. All histological features were characterized with diffuse (≥ 50% of renal cortex) or focal (< 50%) involvement. Interstitial fibrosis and tubular atrophy were graded as mild-moderate (< 50% of cortical area) or severe (> 50%) presentation. In addition, other features looked for in the biopsies were glomerular disease, including sclerosis, ischaemic findings, presence of crescents, capillary loops dilatation with congestion, presence of microthrombi or schistocytes, intersticial infiltrates or presence of acute tubular necrosis (ATN). Histological features like fibrinoid necrosis, mural mixoid thickening, myointimal concentric proliferation, vascular hialinosis, schistocytes or thrombi visualization and arteriosclerosis were considered.
Indirect immunofluorescence staining for Immunoglobulin A, M and G, factor C3, C4 and C1q, albumin, fibrin and light chambers (λ and κ) was performed in all cases. Only biopsies with C4d staining performed by immunofluorescence were considered, with exclusion of immunohistochemical C4d cases (n = 5). C4d deposition was defined as negative (< 10%), focal (10 -50%) or diffuse (> 50%) mural staining of the peritubular capillary network of cortex and medulla.
Considering only samples with at least one glomerulus (n = 17), electron microscopy digital photographs were examined for presence of glomerular endothelial cell swelling with loss of endothelial fenestrations; subendothelial electro -lucent widening in ≥ 10% of glomerular capillaries; glomerular and tubular membrane duplication with/without multilayering (≥ 4 layers) in at least one capillary; PT capilarittis; foot processes effacement and mesangiolysis.
Clinical data regarding demographic features, graft cold ischaemia time, panel reactive antibodies (PRA) at the time of transplantation, HLA mismatch and ABO incompatibility, donor´s age and gender, induction and maintenance immunosuppression (IS) were retrospectively obtained by medical records.
Allograft function and proteinuria at baseline levels (first month after transplantation), at the time of biopsy, 6 months and 1 year post-biopsy were obtained from analytic charts. At the time of biopsy, evidence of haemolytic anaemia, thrombocytopenia, low haptoglobin, high DHL level, presence of schistocytes in blood smear, immunosuppressive therapeutic levels, blood pressure and post-biopsy treatment were obtained. Determination of donor specific antibody (DSA) with HLA phenotype using Class I and Class II screening beds with positives by single -antigen identification (Luminex, One Lamba Inc, Canoga Park, CA) began in our department in 2007 and, during our study period, was requested in 19 cases based on clinical or histological indications.
The data obtained was submitted to statistical analysis using the Statistical Package for Social Sciences (SPSS) 18.0 for Windows (SPSS Inc. Chicago, Illinois). Statistical significance was taken below 5%.
The comparison between continuous variables was made using the students t-test or Wilcoxon test when appropriate. The categorical variables were compared by two-tailed chi -square test or Fishers test. The technique survival analysis at 1 and 5 years was performed using the Kaplan -Meier model with Log-Rank test.
RESULTS
Out of a total of 627 biopsies, 33 cases (6.2%) presented TG features, while 12 patients (2.2%) showed de novo TMA changes. The TG biopsies were performed at later stages (6.9 ± 5.9 vs. 3.5 ± 6.5 years, p = 0.01), while mainly the de novo TMA occurred within the first year (58.3% vs. 18.2%, p = 0.002). However, early TG cases within the first year post -transplatation were diagnosed in six (18.2%) and late de novo TMA diagnosis after two years period occurred in three cases (25%). HCV infection was present only in the TG group and the small number of cases (n = 3) precluded further analysis in this subset of patients.
Comparative analysis between TG and de novo TMA regarding demographic and transplantation features are shown in Table I. De novo TMA patients were older (55.7 ± 9.1 vs. 45.7 ± 13.9 years, p = 0.026) and no significant difference was found in gender, prevalence of diabetes mellitus or hypertension, cause for end -stage renal disease or previous renal transplantation. Active CMV infection was detected in both groups (7.7% vs. 14.3%, p = 0.469).
Type of transplant (deceased or living donor), donor gender, HLA mismatches or ABO incompatibility and induction therapy were similar between both groups. However, the de novo TMA allografts were provided from older patients (53.8 ± 10.1 vs. 41.3 ± 17.9 years, p = 0.035) and a trend to longer cold ischaemia time (1439 ± 210 vs. 1218 ± 441 min, p = 0.141), higher pre -transplant PRA levels (PRA ≥ 25%: 16.7% vs. 3.1%, p = 0.070) and occurrence of previous rejection episodes (21.4% vs. 5.3%, p = 0.079) was found.
Maintenance IS on the TG group consisted entirely of calcineurinic inhibitors. Predominance of tacrolimus therapy was detected in the TG group (84.2% vs. 42.9%, p = 0.002), while no differences were found regarding cyclosporine therapy (15.8% vs. 14.3%, p = 0.921). In addition, mTor inhibitors (42.8%) were only performed in the de novo TMA cases. Pharmacological levels of IS drugs were within the normal therapeutic range in all cases.
Clinical features and laboratory finding comparison between both groups are represented in Table II. Baseline proteinuria was similar between both groups but higher median serum creatinine was observed in the de novo TMA group (sCr 2.1 ± 0.6 vs. 1.5 ± 1.1 mg/dl, p = 0.01) (Table I). At the time of biopsy, microhaematuria (51.7% vs. 15.4%, p = 0.009) and allograft disfunction (sCr 4.9 ± 1.8 vs. 2.7 ± 1.2mg/dl, p < 0.001) were significantly more frequent in de novo TMA when compared to TG, with half of patients under dialysis and none in TG (50% vs. 0%, p < 0.001). On the other hand, TG cases presented higher degree of proteinuria (4.0 ± 3.6 vs. 2.3 ± 1.7 gr/24h, p = 0.02) mainly in the nephrotic range (51.3% vs. 14.3%, p = 0.016).
Systolic and diastolic blood pressure measurements were similar in both groups. Laboratory findings suggestive of haemolytic anaemia (50.0% vs. 5.1%, p < 0.001), thrombocytopenia (85.7% vs. 15.4%, p < 0.001), low haptoglobin (42.9% vs. 5.1%, p < 0.001), presence of schistocytes (21.4% vs. 7.7%, p = 0.001) and higher serum LDH levels (699 ± 591 vs. 397 ± 132 U/L, p = 0.006) were predominately found in the de novo TMA group. However, haemolytic anaemia and low haptoglobin were also observed in two TG cases and presence of schistocytes in three. Nevertheless, these laboratory findings were mainly detected during patient hospitalization before long graft biopsy procedure, whereby the pathologist was unaware of this information.
Comparative description of the histological features present in both groups is described in Table III. At light microscopic (Fig. 1) diffuse glomerular lesions were observed in all de novo TMA cases, opposing to a focal presentation in TG (100% vs. 42.4% p = 0.001). Both groups presented similarities regarding the presence of GMB double contours, glomerulitis, tubulitis and ischaemic features.
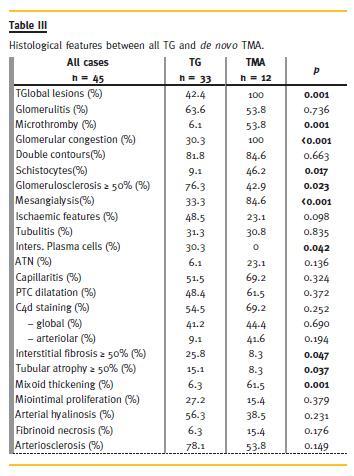
Mesangiolysis was present in both groups but more frequently found in the TMA group (84.6% vs. 33.3%, p < 0.001). Extracapillary proliferation was observed only in TG biopsies (n = 4) and severe glomerulosclerosis, intersticial fibrosis with tubular atrophy were more frequently seen. Interstitial infiltrates formed by macrophages were observed in both groups while the presence of plasma cells was only detected in TG cases (30.3% vs. 0%, p = 0.018). De novo TMA lesions were associated to glomerular capillary congestion (100% vs. 30.3%, p < 0.001), presence of microthrombi (53.8% vs. 6.1%, p = 0.001) and schistocytes (46.2% vs. 9.1%, p = 0.017). These histological features suggestive of TMA were also observed in few early TG cases. No significant difference was found concerning capillaritis or PTC dilatation. Positive C4d detection was slightly more frequent in the de novo TMA group (69.2% vs. 54.5%, p = 0.252) and C4d deposition in arteriolar walls was also predominantly seen in the de novo TMA (41.6% vs. 9.1%, p = 0.042). Detection of DSA was the same in both groups (TG 41.7% vs. TMA 57.1%, p = 0.276). In TG, DSA were mainly anti -HLA Class II (anti - DQ 40%,anti - DR 20%,anti - B 20%,anti A 20%), with mean intensity of 5500 MFI [1000 – 10200 MFI]. Similarly, DSA detection in de novo TMA was mostly constituted by anti -HLA Class II (anti -DQ 75%, anti -B 25%) but with higher mean intensity of 13500 MFI [ 11000-17000 MFI].
No differences were found in myointimal concentric proliferation or vascular hialinosis. De novo TMA cases presented more frequently mural mixoid thickening (61.5% vs. 6.3%, p = 0.001) and, to a lesser extent, fibrinoid necrosis (15.4% vs. 6.3%, p = 0.176).
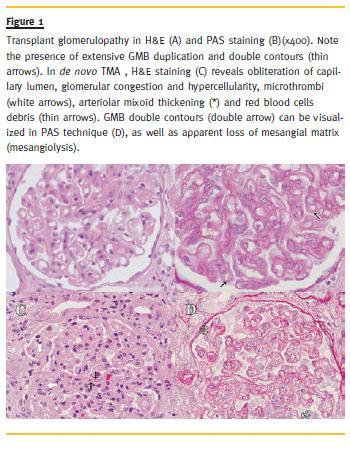
The ultrastrutural features (Fig. 2) of both groups are expressed in Table IV. Only TG cases presented GBM multilayering (75% vs. 0%, p = 0.018) and tended to express more frequently GMB duplication (91.7% vs. 40.0%, p = 0.024), PTCBM thickening (100% vs. 60%, p = 0.074) with multilayering (75% vs. 20%, p = 0.060). Endothelial cell hyperplasia and capilaritis were found in all TG and de novo TMA cases, and no differences in mesangiolysis or foot process effacement were reported.
In the de novo TMA cases, plasmapheresis (78.6% vs. 7.7%, p < 0.001) and IvIG treatment (35.7% vs. 10.3%, p = 0.044) was frequently performed while steroid pulse (35.7% vs. 15.4%, p = 0.134) or rituximab therapy (14.3% vs. 7.7%, p = 0.599) was equallyperformed in both groups (Table II). TMA also motivated higher rates of CNI therapy suspension (42.9% vs. 7.7%, p = 0.007). During the entire follow-up period, graft loss occurred similarly in both groups (47.1% vs. 45.5%, p = 0.926).
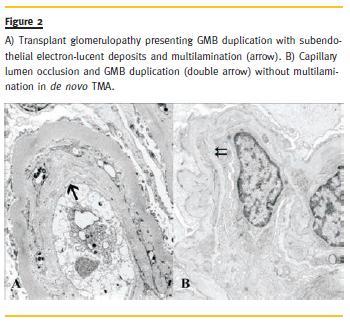
Survival analysis (Fig. 3) revealed lower graft survival in the de novo TMA group at first year post –transplantation (73.3% vs. 97.0% p = 0.013). Regarding the 5–year follow-up, the de novo TMA survival remained stable but still lower than that of TG (73.3% vs. 85.3%, p = 0.082). Graft loss in the de novo TMA cases occurred, on average, within the first month after biopsy, while TG graft loss occurred, on average, 13 months after biopsy (0.72 ± 0.46 vs. 13.2 ± 12.1 months, p = 0.054).
DISCUSSION
Microcirculatory injury can range from acute necrosis to adaptive changes, allowing EC survival but with chronic morphological changes responsible for gradual microvasculature obliteration. Several factors are responsible for microcirculatory aggression in the renal allograft as CMV or HCV infection, ischaemia-reperfusion lesion, calcineurin/mTor inhibitors and humoral rejection2,3.
Acute antibody mediated rejection (AAMR) usually develops in patients with high immunological risk (pre -sensitized or cross -match positive but desensitized patients)7,8. Predominance of microvascular inflammation with EC sweeling, cell necrosis, denudation of basal matrix, platelet aggregation and neuthrophilic infiltration is seen as aggregates of leucocytes (monocytes/macrophages, NK cells, neutrophils) in glomerular (glomerulitis) or PT capillaries (PT capillaritis). Other pathologic features of acute AMR include capillary congestion, vascular thrombi and acute tubular injury (consequence of ischaemic effects on microcirculation in tubular epithelium)9,10.
Late/chronic AMR occurs with de novo DSA or detection of low titres or mean fluorescence intensity of preformed DSA developing later, often in relation to non-adherence11. Sublethal EC injury and domination of microcirculation structural remodelling due to antibody -mediated injury are found, which originate new basement membrane deposition (duplication) or multilamination of the existing capillary basement membrane beneath the injured endothelium. Episodic or subclinical alloantibody-mediated endothelial injury causes time-dependent structural changes in microvessels and arteries, tubular atrophy with interstitial fibrosis and glomerulosclerosis12. Additionally, EC activation may lead to pro -thrombotic and pro-inflamatory changes, manifested by acute/chronic thrombotic microangiopathy and various degrees of glomerulitis and capilaritis13.
Transplant glomerulopathy is believed to be the final manifestation of chronic capillary injury that begins right after transplantation. Rarely diagnosed during first year, several ultrastrutural studies based on protocol biopsies in well-functioning renal allograft revealed features representative of overt TG within the first 3 months after transplantation14,15. The endothelial cell injury is expressed focally and is characterized by glomerular endothelial cell swelling with loss of fenestration, mesangial cell swelling with matrix expansion and subendothelial electron-lucent widening GMB. Glomerular basement membrane duplication and lamination, typical findings in later TG, seem to result from chronic endothelial lesion, with acute injury events followed by endothelial cell accommodation. GMB remodelling occurs with expansion of lamina rara interna and deposition of finely fibrilary or flocullent material in a subendothelial location. This capillary injury is not limited to glomerular capillaries but also affects PTC with subsequent multilamination of the basement membrane15.
Transplant glomerulopathy and the closely related lesions of chronic capillary remodelling are felt to be manifestations of CAMR. These changes are known to be associated to detection of antibodies against most notably HLA class II antigens (expressed in glomerular and pericapillary endothelial cells), as well as C4d staining and leucocyte aggregates in glomerular and PT capillaries16-18. In addition, early TG cases have numerous similarities with AAMR episodes, regarding histological (glomerulitis and/or capilaritis), immunological (C4d in PTC) and serologic (DSA detection) findings. Thus, the presence of glomerular endothelial cell swelling, subendothelial widening without electro -dense deposits and GBM duplication on renal allograft biopsy may be related to smoldering AMR and are likely to represent precursor lesions of overt TG19.
Studies performed with C4d staining reported that AMR is common (about 55% of episodes) and, therefore, an important cause of de novo TMA6,20,21. Thus, de novo TMA manifestation has been noted in 4-46% of patients with AMR22. It usually occurs in the early period post -transplantation and presents immunopathologic features of acute/early AMR including neutrophilic glomerulitis and peritubular capilaritis, fibrinoid necrosis, glomerular thrombi, mensagiolysis, GBM duplication and PTC C4d deposition. Detection of DSA is frequent and mostly constituted by anti-HLA II, similar to TG. Laboratory features of TMA occur in a variable manner, but are more frequently found in the earlier cases. Evidence for haemolytic anaemia (low haptoglobin, elevated DHL and schistocytes) and thrombocytopenia are more frequent in C4d positive cases, suggesting severe endothelial damage secondary to increased levels of inflammatory mediators released in antibody -mediated injury22. Smoldering cases of de novo TMA also occur and are detected in later stages (2 -6 years after transplantation), presenting only a progressive graft dysfunction often associated with arterial hypertension23.
Some authors defend that TG may in fact represent this chronic smoldering glomerular form of TMA6.
Typical laboratory features of TMA are usually absent and histological features may include severe vascular injury, with prominent mucoid arterial/arteriolar intimal thickening and endothelial swelling with GBM double contours.In our study, patients were submitted to renal biopsy based on clinical indications. The TG diagnosis was performed in long-term renal allografts with insidious graft dysfunction and increasing proteinuria, half on nephrotic range. Curiously, clinically TG cases within the first year post -tranplantation (15%) were found. This earlier fraction would probably be higher if protocol biopsies were performed, given the insidious clinical presentation of TG. Thus, the reported incidence of this disease is probably an underestimate of its true value.
De novo TMA diagnoses were mostly performed within the first year after transplantation in patients presenting severe graft failure (half of them on temporary dialysis) and laboratory features of TMA at the time of biopsy. These laboratory findings were also found in the early TG cases suggesting that, although expressing less intensity than de novo TMA, early cases seem to mimic some of its clinical features. Patients who developed de novo TMA tended to present higher PRA levels and previous rejection episodes than TG cases. This higher immunological risk may be a relevant predisposing factor for post -transplant de novo TMA involving renal allograft, reinforcing the hypothesis that early/acute AMR may be the initial pathological mechanism.
Histopathologic features were similar between both diseases. Typical findings in TG, as GMB thickening and double contours, were equally found in de novo TMA. While TG presented evidence of advanced chronic damage (with glomerulosclerosis, intersticial fibrosis and tubular atrophy), acute and intense endothelial reactivity with diffuse capillary congestion was found in de novo TMA. This diffuse endothelial inflammatory injury with neutrophilic adhesion may predispose to microvascular thrombosis, explaining our higher frequency of capillary thrombi in TMA, as reported in previous studies22.
Mesangium injury was also detected in both situations.
Mesangiolysis results from accumulation of pale material, reticulation and oedema leading to disruption of the matrix. Usually described in ultrastructural analysis of TG23, mesangiolysis was easily visualized in de novo TMA light microscopy, where mesangial cell activation is more evident and the dissociation between the apparent abundant mesangial matrix in haematoxylin -eosine technique not confirmed in PAS technique may result from intense intracellular oedema and matrix breakdown related to the intense inflammatory injury24.
The similarities between these pathologies made it difficult to distinguish TG or TMA base only on optical findings, since they presented overlapping features in a proportion of cases: some TMA cases did not shown characteristic glomerular microthromby or laboratory evidence of haemolytic anaemia while some TG biopsies presented glomerular microthromby, glomerular congestion or laboratory evidence for haemolysis. In these doubtful samples, true definition of TG or TMA was only achieved after ultrastructural analysis and based on detection of endothelial multilayering.
Electron microscopic analysis showed that GBM and PTCBM multilayering were predominately found in TG cases. These findings sustain the hypothesis of periodic subclinical endothelial lesion, mostly cause by varying circulation antibody levels, supported by previous description of intermittent C4d staining in TG cases25. Logically, we can presume that TG may represent a chronic sub-lethal endothelial cell injury, with subsequent cell activation and repair, leading to GBM and PTCBM remodelling26. While TG results from endothelial activation by injury mechanisms leading to increased protein synthesis, de novo TMA may represent a severe EC injury with complement -mediated lysis without remodelling processes, explaining the absence of remodelling related features in our results.
Typical TMA arteriolar findings characterized by intimal mixoid thickening were found in most our biopsies, with severe occlusion of vascular lumen and presence of fragmented red blood cells. In our TG cases, vascular damage was characterized as pronounced concentric and intimal fibrous thickening, presenting different evolutional stages with progressive narrowing of vascular lumen. Both lesions can gradually evolve in arteriolar obliteration, leading to tissue underperfusion and fibrosis. Ischaemic morphologic features, like Bowman´s space dilatation and glomerular tufts collapse with GBM wrinkling, were frequently found in TG case, possibly due to their chronic evolution. These same features were also observed occasionally in de novo TMA but, in these cases, acute obliteration of capillaries occurred, explaining why ischaemic ATN and fibrinoid necrosis were frequently found.
The association between PTC binding of the complement product of the classical pathway (C4d) and active antibody-mediated rejection is well established25,27.
Thus, C4d detection may be regarded as an indirect sign of alloantibody response, acting like a foot print of a damaging event, supporting the hypothesis that alloantibodies play an important role.
Detection of pre -transplant anti -HLA class II antibodies has also shown to increase the risk of developing TG, with consequent poor graft outcome25,28. In previous studies, TG positive PT C4d staining varied between 22-62%, with strong association with detection of DSA anti -HLA II27,29. Our study revealed DSA detection in five of 12 patients (41.7%), mostly anti HLA class II (60%). These results would probably be more representative if DSA detection was performed in all cases.
Satoskar et al.30 demonstrated PTC C4d staining in 55% de novo TMA cases. Moreover, DSA was detected in 50% of those patients, 80% of those formed by anti -HLA class II. Expressive PTC C4d staining was detected in our de novo TMA cases (69.2 %), with similar detection of DSA (57.1%) mostly anti -HLA class II (75%). These similarities in immunological features between TG and de novo TMA reinforces the hypothesis that both share endothelial injury, with consequent capillaritis. Slightly higher expression in PTC C4d staining in de novo TMA found in our study, when compared to TG, may be related to a broader AMR involvement in pre-sensitized patients. Participation of immunological mechanisms responsible for pro-thrombotic factors release could justify the higher presence of capillary thrombi in these cases. Interestingly, our results demonstrated an association between arteriolar C4d deposition with de novo TMA. Generally considered as a non -specific finding, Collins et al.31 previously reported C4d staining in arteriolar wall in cases of fibrinoid necrosis associated to early/acute AMR. Immunological rejection in arterial endothelium is known to be less dependent in complement activation, since predominately T-cell-mediated mechanisms via NK cells are present, explaining the lack of arterial C4d deposition in general cases32,33.
However, since fibrinoid necrosis is also a histological marker of early/acute AMR, we hypothesise that, within acute and severe cases, antibody complement activation plays major role for endothelial arteriolar damage, with consequent C4d deposition.
The C4d detection is recently thought to be a less sensitive marker for AMR than previously thought, especially in chronic AMR. A previous report showed a dissociation between C4d deposition and evidence of AMR in TG: in cases with endothelial activation and injury (detected by high mRNA endothelial gene expression) in combination of chronic AMR histological features (capilaritis, glomerulitis and interstiticial fibrosis/tubular atrophy), only 40% were C4d positive19.
Accordingly, our result regarding to C4d positive TG cases may reflect an underestimation of the true prevalence of CAMR.
On the other hand, other non -alloantibody mediated processes may be implied on pathogenesis of TG, especially HCV infection and TMA3. Other studies also showed overlapping features of CMV infection with TG and TMA34. In our study, sub -analysis regarding chronic HCV infection was not made because of the small number of cases, and no difference was found in active CMV infection between both groups.
Factors related to immunosuppression may also be implied on the aetiology of both entities, particularly TMA. A cyclosporine endothelial lesion may lead to TMA due to vasoconstrictor, prothrombotic and pro-necrotic activity, with subsequent mucinoid thickening of arteriolar intima or nodular hialinosis35. No difference was found between TG and de novo TMA regarding CSA therapy at the time of biopsy and mTOR inhibitors therapy was only found in the TMA group. Additionally, a tendency to longer graft cold ischaemia time was found in this group. The high incidence of ischaemia-reperfusion injury in these cases can lead to severe endothelial damage, with surface antigen presentation and potentiating humoral rejection mechanisms. Combining this finding with the high PTC C4d staining detected we can presume that humoral rejection was responsible for most of our de novo TMA cases. Nevertheless, cyclosporine and mTOR inhibitor therapy has also an additive effect on endothelial injury.
Interstitial inflammation with tubulitis was equally found in both pathologies. In addition to polymorphonuclear neutrophils and lymphocytes, plasma cells were detected only in TG cases. Several studies have correlated the presence of plasma cells with C4d deposition and DSA36,37. Frequently found in older grafts, they are usually located near peritubular capillaries and are probably related with chronic humoral rejection. Intragraft production of DSA has been demonstrated and the subsequent chronic immune response induces continuous B -cell stimulation leading to posterior differentiation into alloreactive plasma cells37. Exclusive plasma cells presence in TG and not in de novo TMA in our study suggests that chronic humoral stimulation is required to plasma cell differentiation.
Evidence of humoral rejection with morphological and clinical features of TMA justified plasmapheresis in the majority of our patients, with graft loss occurring in four of 11 patients (36.3%), similar to Satoskars results (35%) 29. While graft loss occurred equally in both diseases, survival analysis revealed that graft loss in de novo MAT occurred earlier, with lower survival rate at 1 year and, in only one case, graft loss occurred beyond this hallmark, suggesting that successful treatment in early stages may benefit graft survival. As regards TG, graft loss within the first 5 years post -transplantation occurred in only four cases (26.7%), reflecting its indolent evolution.
However, the survival time of renal graft after biopsy procedure was relatively short in these cases (on average 1 year). This is explained by the fact that allograft biopsy was performed based on clinical indication. At this point, chronic TG reveals advanced glomerulosclerosis, interstitial fibrosis and tubular atrophy, as confirmed in our results. Accordingly, the late diagnosis with irreversible allograft damage explains the short survival after diagnosis.
To our knowledge, this is the first study with comparative analysis between TG and de novo TMA.
Nevertheless, our analysis was performed in a retrospective manner so general conclusions cannot be extrapolated. The small number of cases evolved from a single centre, the absence of DSA determination in all patients and biopsies performed only by clinical indications may also have contributed to reduced statistical significance. Additionally, the small number of cases with ultrastructural analysis is another drawback.
In summary, our finding suggests that TG and de novo TMA represent manifestations within the spectrum of multifactorial microcirculation injury and differentiation between them, when based on pathologic features, is usually challenging, expressing resemblances in histological and ultrastructural changes secondary to the initial and/or persistent endothelial lesion. Besides infectious and pharmacologic endothelial injury, antibody mediated rejection with complement activation has a major role on their pathogenesis since C4d staining occurs and presence of DSA is detected in the majority of cases. While
TG is the result of subclinical ongoing antibody mediated injury that begins right after transplantation, presenting progressive cyclic EC lesions and accommodation, de novo TMA resembles an acute alloantibody -mediated rejection, suggesting that severe and broader humoral mechanisms in pre-sensitized patients are responsible for earlier presentation and premature graft loss. Further investigation in early TG cases detected by protocol biopsies and correlation with humoral rejection mechanisms will allow better understanding of the pathologic lesions shared by both manifestations. Additionally, electron microscopy is a relevant tool with important diagnostic value in the evaluation of the pathogenesis of endothelial injury in chronic allograft biopsies.
References
1. Cosio FG, Grande JP, Wadei H, Larson TS, Griffin MD, Stegall MD. Predicting subsequent decline in kidney allograft function from early surveillance biopsies. Am J Transplant 2005;5(10):2464 -2472. [ Links ]
2. Cosio FG, Gloor JM, Sethi S, Stegall MD. Transplant glomerulopahy. Am J of Transplant 2008;8(3):492 -496. [ Links ]
3. Baid -Agrawal S, Farris AB 3rd, Pascual M, et al. Overlapping pathways to transplant glomerulopathy: chronic humoral rejection, hepatitis C infection, and thrombotic microangiopathy. Kidney Int 2011;80(8):879 -885. [ Links ]
4. Schwimmer J, Nadasky TA, Spitalnik PF, Kaplan KL, Zand MS. De novo thrombotic microangiopathy in renal transplant recipients: A comparison of hemolytic uremic syndrome with localized renal thrombotic microangiopathy. Am J Kidney Dis 2003;41(2):471 -479. [ Links ]
5. Ponticelli C, Banfi G. Thrombotic microangiopathy after kidney transplantation. Transplant Int 2006;19(10):789 -794. [ Links ]
6. Laszik ZG, Silva FG. Hemolytic uremic syndrome, thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. In: Jennette JC, Olson JL, Schwartz MM, Silva FG, eds. Heptinstall´s pathology of the kidneys, 6th ed, Philadelphia: Lippincott Williams & Wilkins, 2007;701 -764. [ Links ]
7. Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant 2008;8(4):753 -760. [ Links ]
8. Sis B. Endothelial molecules decipher the mechanisms and functional pathways in antibody -mediated rejection. Hum Immunol 2012;73(12):1218 -1225. [ Links ]
9. Magil AB, Tinckam K. Monocytes and peritubular capillary C4d deposition in acute renal allograft rejection. Kidney Int 2003;63(5):1888 -93. [ Links ]
10. Sis B, Jhangri GS, Riopel J, et al. A new diagnostic algorithm for antibody –mediated microcirculation inflammation in kidney transplants. Am J Transplant 2012;12(5):1169-1179. [ Links ]
11. Sellares J, de Freitas DG, Mengel M, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and non-adherence. Am J Transplant 2012;12(2):388-399. [ Links ]
12. Colvin RB. Antibody -mediated renal allograft rejection: diagnosis and pathogenesis. J Am Soc Nephrol 2007;18(4):1046 -1056. [ Links ]
13. Drachenberg CB, Papadimitriou JC. Endothelial injury in renal antibody-mediated allograft rejection: a schematic view based on pathogenesis. Transplantation 2013;95(9):1073 -1083. [ Links ]
14. Wavamunno MD, O´Connell PJ, Vitalone M, et al. Transplant glomerulopathy: ultrastructural abnormalities occur early in longitudinal analysis of protocol biopsies. Am J Transplant 2007;7(12):2757-2768. [ Links ]
15. Ivanyi B. Transplant capillaropathy and transplant glomerulopathy: ultrastructural markers of chronic renal allograft rejection. Nephrol Dial Transplant 2003;18(4):655-660. [ Links ]
16. Haas M, Mirocha J. Early ultrastructural changes in renal allografts: correlation with antibody -mediated rejection and transplant glomerulopathy. Am J Transplant 2011;11(10):2123 -2131. [ Links ]
17. Regele H, Böohmig GA, Habicht A,.et al. Capillary deposition of complemente split product C4d in renal allografts is associated with basement membrane injury in peritubular and glomerular capillaries: A contribution of humoral immunity to chronic allograft rejection. J Am Soc Nephrol 2002;13(9):2371 -2380. [ Links ]
18. Silva C, Cotovio P, Marques M, et al. Transplant glomerulopathy: clinico–pathologic features. Port J Nephrol Hypert 2013;27(3):209 -215. [ Links ]
19. Sis B, Jhangri GS, Bunnag S, Allanach K, Kaplan B, Halloran PF. Endothelial gene expression in kidney transplants with alloantibody indicates antibody -mediated damage despite lack of C4d staining. Am J Transplant 2009;9(10):2312 -2323. [ Links ]
20. Nickeleit V, Zeiler M, Gudat F, Thiel G, Mihatsch MJ. Detection of the complement degradation product C4d in renal allografts: Diagnostic and therapeutic implications. J Am Soc Nephrol 2002;13(1):242-251. [ Links ]
21. Lefaucher C, Nochy D, Hill GS, et al. Determinants of poor graft outcome in patients with antibody-mediated acute rejection. Am J Transplant 2007;7(4):832-841. [ Links ]
22. Meehan SM, Kremer J, Farah NA, et al. Thrombotic microangiopathy and peritubular capillary C4d expression in renal allograft biopsies. Clin J Am Soc Nephrol 2011;6(2):395-403. [ Links ]
23. Karthikeyan V, Parasuraman R, Shav V, Vera E, Venkat KK. Outcome of plasma exchange therapy in thrombotic microangiopathy after renal transplantation. Am J Transplant 2003;3(10):1289-1294. [ Links ]
24. Morita T, Churg J. Mesangiolysis. Kidney Int 1983;24(1):1-9. [ Links ]
25. Fotheringham J, Angel CA, McKane W. Transplant glomerulopathy: morphology, associations and mechanism. Nephron Clin Pract 2009;113(1):c1-c7. [ Links ]
26. Husain S, Sis B. Advances in the understanding of transplant glomerulopathy. Am J Kidney Dis 2013;62(2): 352-363. [ Links ]
27. Gloor JM, Sethi S, Stegall MD, et al. Transplant glomerulopathy: subclinical incidence and association with alloantibody. Am J Transplant 2007;7(9):2124-2132. [ Links ]
28. Colvin RB. Pathology of chronic humoral rejection. Contrib Nephrol 2009;162:75-86. [ Links ]
29. Sis B, Campbell PM, Mueller T, et al. Transplant glomerulopahy, late antibody –mediated rejection and the ABCD tetrad in kidney allograft biopsies for cause. Am J Transplant 2007;7(7):1743-1752. [ Links ]
30. Satoskar AA, Pelletier R, Adams P, et al. De novo thrombotic microangiopathy in renal allograft biopsies–role of antibody -mediated rejection. Am J Transplant 2010;10(8):1804-1811. [ Links ]
31. Collins AB, Schneeberger EE, Pascual M, et al. Complement activation in acute renal allograft rejection: diagnostic significance of C4d deposits in peritubular capillaries. J Am Soc Nephrol 1999;10(10):2208-2214. [ Links ]
32. Hirohashi T, Uehara S, Chase C, et al. Complement independent antibody-mediated endarteritis and transplant arteriopathy in mice. Am J Transplant 2010;10(3):510-517 [ Links ]
33. Smith RN, Kawai T, Boskovic S, et al. Four stages and lack of stable accommodation in chronic alloantibody -mediated renal allograft rejection in Cynomolgus monkeys. Am J Transplant 2008;8(8):1662-1672. [ Links ]
34. Keyzer K, Van Laecke S, Peeters P, Vanholder R. De novo Thrombotic microangiopathy induced by cytomegalovirus infection leading to renal allograft loss. Am J Nephrol 2010;32(5):491-496. [ Links ]
35. Mihatsch MJ, Kyo M, Morozumi K et al. The side-effects of cyclosporine-A and tacrolimus. Clin Nephrol 1998;49(6):356 -363. [ Links ]
36. Poduval RD, Kadambi PV, Josephson MA, et al. Implications of immunohistochemical detection of C4d along peritubular capillaries in late acute renal allograft rejection. Transplantation 2005;79(2):228-235. [ Links ]
37. Thaunat O, Nicoletti A. Lymphoid neogenesis in chronic rejection. Curr Opin Organ Transplant 2008;13(1):16-19. [ Links ]
Dr. Rui M. Costa
Nephrology Department
Centro Hospitalar de Trás-os-Montes e Alto Douro
Avenida da Noruega, 5000 -508 Lordel
E-mail: ruimiguelccosta@gmail.com
Conflict of interest statement: None declared.
Received for publication: 21/04/2014
Accepted in revised form: 09/07/2014














