Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.28 no.2 Lisboa jun. 2014
ORIGINAL ARTICLE
Anaemia in the first year after paediatric renal transplant
Anemia no primeiro ano após transplantação renal pediátrica
Sara Ferreira1, Sara Aguilar1, Ana Rita Sandes1, Teresa Rodrigues2, Carla Simao1, Margarida Almeida1
1Paediatric Nephrology Unit, Paediatrics Department - Hospital de Santa Maria. Centro Hospitalar de Lisboa Norte, EPE. Lisboa, Portugal.
2Biomathematic Laboratory -Faculdade de Medicina de Lisboa. Lisboa,Portugal.
ABSTRACT Introduction: Anaemia after renal transplantation has been associated with chronic graft dysfunction and increased cardiovascular mortality. There are few paediatric studies on the prevalence and on the aetiological factors involved. Objectives: To determine the prevalence of anaemia in children after renal transplantation (RT) and associated risk factors. Methods: A descriptive, analytical, retrospective study was conducted by consulting the records of patients followed in the Paediatric Nephrology Unit of tertiary care hospital in Lisbon, with more than one year of renal transplantation. The prevalence of anaemia was determined at 3 and at 12 months post -RT and the following potential risk factors were analysed: pre -renal transplantation anaemia, viral infections (CMV, EBV, PVB19), urinary tract infections, iron deficiency, graft function and occurrence of acute graft dysfunction episodes. Results: We evaluated 45 children: 82% of Caucasians, 58% female, mean age at transplant 9 ± 3.9 years. Anaemia was present in 31 patients at 3 months after RT (71%) and in 30 patients at 12 months post -RT (67%). At pre-RT 29 children had anaemia (64%). At 12 months post -RT the anaemic group had: pre-RT anaemia in 70%, reduced glomerular filtration rate in 57%, viral infections in 50% (mainly CMV), graft dysfunction in 29%, iron deficiency in 26% and recurrent urinary infections in 12%. Viral infection was the only statistically significant factor at the 12 months analysis (p = 0.03). Conclusion: The prevalence of anaemia was relevant in our centre, 67% at 12 months post-RT with a multifactorial aetiology. Viral infection was the only statistically significant associated factor. Thus, regular screening of post -transplantation anaemia and evaluation of the multiple risk factors associated is recommended. Anaemia should be properly assessed and treated.
Key-words: Anaemia; paediatric renal transplantation.
RESUMO
Introdução: A existência de anemia apos transplantação renal tem sido associada a disfunção cronica do enxerto e aumento da mortalidade por eventos cardiovasculares. Existem ainda poucos estudos em idade pediátrica sobre a prevalência de anemia e os fatores etiológicos da mesma. Objetivos: Determinar a prevalência de anemia apos transplantação renal (TR) e os possíveis fatores associados. Métodos: Foi efetuado um estudo descritivo, analítico, retrospetivo através da consulta de processos clínicos de doentes com mais de um ano apos transplantação renal, seguidos na Unidade de Nefrologia Pediátrica de um centro terciário em Lisboa. Determinamos a prevalência de anemia aos 3 e aos 12 meses apos transplantação e analisamos os seguintes fatores de risco: existência de anemia pré-transplantação, infeções virais (CMV, EBV, PVB19), infeções do trato urinário, ferropenia, função do enxerto e episódios de disfunção aguda do mesmo. Resultados: Foram avaliadas 45 crianças: 82% caucasianos, 58% do sexo feminino, com idade media a data do transplante de 9 ± 3,9 anos. Verificou-se existência de anemia em 31 crianças (71%) 3 meses apos a TR e em 30 crianças (67%) 12 meses apos TR. No pré TR 29 crianças (64%) apresentavam anemia. No grupo com anemia aos 12 meses apos TR verificou -se a presença de: anemia pre TR em 70%, redução na taxa de filtração glomerular em 57%, infeções virais em 50% (principalmente CMV), disfunção do enxerto em 29%, deficiência de ferro em 26% e infeções urinarias recorrentes em 12%. A infeção viral foi o único fator estatisticamente significativo na analise aos 12 meses (p = 0,03). Conclusão: No nosso centro a prevalência de anemia aos 12 meses apos transplantação renal foi elevada (67%) e de etiologia multifatorial. A infeção viral foi o único fator estatisticamente significativo associado a existência de anemia. Recomenda-se o rastreio regular de anemia bem como dos múltiplos fatores de risco associados. A anemia apos transplantação renal deve ser devidamente avaliada e tratada.
Palavras-Chave:Anemia; transplantação renal pediátrica.
INTRODUCTION
Renal transplantation (RT) is the therapy of choice in end-stage kidney disease in children. Improved strategies for monitoring transplant patients, including the evolution of immunosuppressive therapy, have led to an increase in patients and graft survival. There is now a greater awareness of other morbidity factors, which contribute significantly to the reduction of patients quality of life and to premature mortality.
Anaemia is independently associated with increased mortality and morbidity, including decreased cognitive ability and commitment of growth, as well as left ventricular hypertrophy, which in adults is an independent factor of mortality1,2. Chronic allograft nephropathy is also significantly higher in anaemic patients3 and 12-month post-transplantation anaemia (PTA) has been associated with subsequent graft loss and patient mortality4.
There are few reports on the prevalence of anaemia and associated risk factors in paediatric renal transplant recipients and much of our understanding of PTA is based on studies of adult transplant recipients.
The limited number of paediatric studies report a prevalence of 25% to 83%1. This variability is mainly because there is no consensual definition for anaemia and iron deficiency in this special population5.
Anaemia may occur at any time following transplantation.
Its cause is multifactorial. In the immediate post-operative period we must consider pre-RT anaemia management, impaired erythropoietin production or release by the transplanted kidney and blood loss.
Lately other risk factors include: the use of immunosuppressive agents and other farmacological agents with direct antiproliferative effects on the bone marrow6, viral infections (cytomegalovirus (CMV), Epstein-Barr virus (EBV) and parvovirus B19 (PVB19)), bacterial infections, rejection episodes7,8, the use of angiotensin converting enzyme inhibitors (ACE)11, insufficient diet resulting in iron, vitamin B12 and folate deficiency and chronic allograft nephropathy.
OBJECTIVES
The aim of this study was to establish the prevalence of anaemia in paediatric renal transplant recipients at 3 months and at 12 months after renal transplantation and to identify associated risk factors in a single paediatric centre.
MATERIAL AND METHODS
A retrospective descriptive and analytical study was conducted by consulting the medical files of patients with, at least, 12 months of renal transplantationactively followed in the Paediatric Nephrology Unit of Hospital de Santa Maria, a tertiary care Hospital in Lisbon.
The following data were analysed: demographic characteristics, pre-transplantation haemoglobin (Hb) level, immunosuppressive regimen, use of angiotensin converting enzyme inhibitors (ACEI), 3 and 12 months post-transplant: haemoglobin level, mean corpuscular volume (MCV), mean haemoglobin level (HGM), occurrence of viral infections (CMV, EBV, PVB19), urinary tract infections (UTI), iron deficiency, graft function and occurrence of acute graft dysfunction episodes.
Anaemia was defined as a haematocrit level more than two standard deviations (SD) below published means for age or as erythropoietin (EPO) dependency to maintain haematocrit at a normal level.
Hypochromia and microcitosis cut-off levels were defined if VGM or HGM levels were two standard deviations below the mean expected for age and sex.
Pre-renal transplant anaemia was defined using de KDIGO diagnostic criteria of anaemia in children: Hb concentration: < 11.0 g/dl in children 0.5–5 years; < 11.5 g/dl in children 5–12 years; < 12.0 g/dl in children 12–15 years.
Iron deficiency was defined by serum ferritin < 30 mg/l.
CMV Infection was defined by the presence of more than 5000 CMV DNA copies/ml serum, EBV infection by the identification of more than 10 DNA copies/ml serum and PVB19 infection when detected > 100.000 DNA copies/ml serum, using the protein chain reaction method.
Urinary tract infection was defined as growth of ≥ 100,000 colony forming units (CFU)/mL in urine culture and only more than 3 UTI were considered.
Delayed graft function was defined when dialysis was performed during the first week after transplantation.
Acute graft dysfunction was considered for the elevation of creatinine level more than 20% above baseline in at least two consecutive laboratory assessments and only more than one episode after RT was considered.
Glomerular filtration rate (GFR) was calculated according to the Schwartz formula8.
The K/DOQI workgroup classification system was used to determine the level of renal function9.
All patients were transplanted between 1995 and 2011 and were, at least, 12 months post-transplantation at the time of study.
Statistical analysis was performed using IBMRSPSSR version 21 software. The analysis of association was done for qualitative variables using Fishers exact test for 2 by 2 tables when at least one expected frequency was less than 5 and Pearson chi-squared in the other cases. A p -value of less than 0.05 was defined as statistically significant. The strength of association was studied with odds ratio (OR) with a confidence interval of 95% (CI 95%).
RESULTS
Forty -five children were enrolled, 26 (58%) female and 37 (82%) Caucasian. Patients characteristics are summarized in Table I. The mean age at transplantation was 9 ± 3.9 years. In 44 children (98%), it was the first transplant and deceased donor was the source in 41 children (91%). Two patients had delayed graft function.
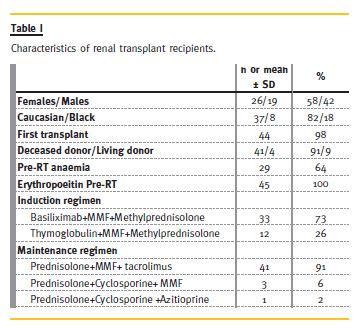
Immunosuppression included induction therapy with anti-CD25 monoclonal antibody or thymoglobulin, combined with mycophenolate mofetil (MMF) and methylprednisolone. Maintenance scheme was prednisolone, tacrolimus and MMF in the majority of patients (91%). Only 9% had a different maintenance scheme: prednisolone and cyclosporine combined with MMF or prednisolone and azathioprine combined with cyclosporine.
Pre-RT aanemia was present in 29 patients (64%) despite all being on erythropoiesis-stimulating agents therapy.
All patients had cotrimoxazol prophylaxis for Pneumocystis jiroveci for the first 6 months after renal transplantation and the majority was on valganciclovir 3 to 6 months after RT. Only three patients (6%) were treated with ACEI inhibitors.
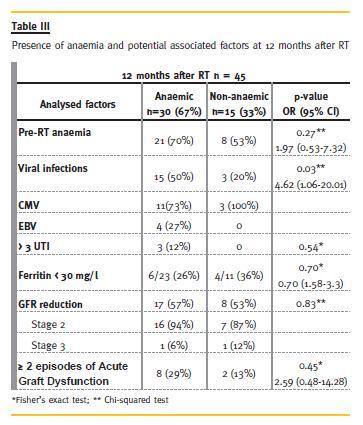
The prevalence of anaemia and of the potential associated factors analysed at 3 and at 12 months is shown in Table II and Table III, respectively.
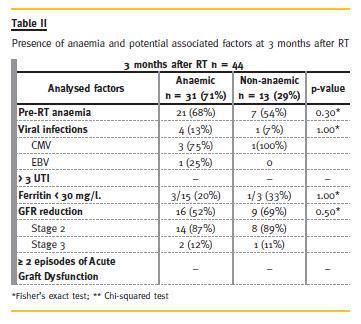
The prevalence of anaemia was 71% (n = 31) 3 months after RT (71%, 95% CI: 56% -84%), and 67% (n = 30) 12 months after RT (67%, 95% CI: 52%-81%). At 3 months post -transplant most patients (74%) had normochromic normocytic anaemia and at 12 months 50% of patients had normochromic normocytic anaemia.
Viral infections were more frequent in the anaemic group, both at 3 and at 12 months, mainly CMV infection. This difference was statistically significant at the 12 months analysis (p = 0.03). Parvovirus was not detected during the first 12 months after RT.
Concerning other analysed factors, at 12 months post-RT, the anaemic group had more frequently pre-RT anaemia, GFR reduction, more than 3 UTIs/year and more episodes of acute graft dysfunction, although these differences did not reach statistical significance in our population.
DISCUSSION
Our results are consistent with others confirming a high prevalence of post RT anaemia in children.
Yorgin et al. in a cohort study of 162 paediatric transplant recipients defined anaemia as a haematocrit level more than 2SD below published means for age or as erythropoietin dependency to maintain haematocrit at a normal level. Sixty -seven per cent of recipients were anaemic at the time of transplant and from 6 to 60 months post-RT the prevalence of anaemia was 64.2% to 84%10. Mitsnefes et al. found anaemia was present in 25.5% of their paediatric patients 1 year after RT. In this study, anaemia was defined using the KDOQI clinical practice guidelines for anaemia of chronic kidney disease11.
We found more than 2/3 of patients (71%) with anaemia at 3 months PT and this value remained elevated at 12 months (67%).
Most patients had normochromic normocytic anaemia, which is the type found in chronic diseases. It may be found in a variety of inflammatory conditions and its cause is multifactorial, involving abnormalities in iron use, a decrease life span of erythrocytes, direct inhibition of haematopoiesis and a relative deficiency of erythropoietin12.
In the first 3 months PT, the immediate known risk factors that cause anaemia include iatrogenic losses (surgery, phlebotomy), presence of anaemia before RT, delayed graft function and discontinuation of treatment with EPO (since there is often a low production of erythropoietin by the graft)13,14. As is known, if chronic EPO therapy is suddenly stopped the recovery of haemoglobin level may be more prolonged than if the dose is gradually tapered.
Episodes of acute graft dysfunction, viral infection (CMV, EBV, and PVB19), iron deficiency, immunosuppressive medication, chronic medication with ACE inhibitors and receptor antagonists of angiotensin II, and number of kidney transplants have also been implicated as factors potentially associated with post RT anaemia15.
Although we have examined several different anaemia-associated factors, only the occurrence of viral infections was statistically significant. Viral infections, namely CMV infection, which is thought to infect bone marrow stromal cells, were an important contributing factor, probably not only by the infection itself but also because of the myelotoxicity of the antiviral therapy.
Probably due to the small sample size, other analysed aspects did not reach statistical significance.
However, there were several other factors more frequent in the anaemic patients, which certainly contributed to anaemia, including reduced GFR, recurrent UTIs and episodes of acute graft dysfunction.
Concerning iron deficiency, the ideal target levels for iron status in renal transplant patients is not consensual. There is also controversy about the ideal markers for iron deficiency in this population. Some authors indicate that the values should be those defined for chronic paediatric kidney disease16. The more consensual markers are serum ferritin and transferrin saturation, however, other studies have shown serum iron as the best predictor of anaemia 17,18. In our study, we considered ferropenia when the ferritin level was below the value accepted for the general paediatric population. Probably, if we had used the values for CKD the prevalence of ferropenia it would have been much higher. Of notice, at 3 months 20% of the patients with iron deficiency had anaemia and at 12 months this percentage reached 26%. There is no statistical significance due to the small sample and due to an elevated missing data regarding ferritin in the non-anaemic group.
We registered an elevated rate of recurrent UTIs, which may have contributed to an inflammatory state that also leads to anaemia. This context adds difficulty to value ferritin level as it can be raised in the presence of inflammation. UTIs also can be responsible for anaemia due to graft dysfunction.
Regarding graft function in the anaemic group, 29% had more than two acute graft dysfunction episodes at 12 months and 57% had GFR reduction.
Delayed graft function, acute or chronic dysfunction may cause damage or dysregulation of EPO production.
Thus, in these patients, the reduction of erythropoietin production by the graft may contribute to explain the anaemia, especially in those with no other factors identified.
Recent reviews have demonstrated that erythropoietin resistance and/or deficiency exist in transplanted patients, even in those without apparent graft failure. This may support managing post-RT anaemia in the same manner as in chronic renal failure. It was also demonstrated a peak of erythropoietin production by 3 months after RT which should allow stable haemoglobin levels during the first 2-6 months after RT19. Thus, some authors defend the institution of therapy with erythropoietin in patients who are not able to maintain normal haemoglobin 6 months after RT20,21. In a recent review20 it is suggested the use of iron and EPO therapy in the post -RT period, avoiding blood transfusions among these patients due to the high prevalence of PTA.
Nevertheless, few randomized studies have specifically addressed the efficacy and safety of EPO therapy in kidney transplant patients, particularly paediatric patients.
It would have been appropriate to register erythropoietin levels in our patients, however, this was a retrospective study and assay of erythropoietin is not a routine practice in our unity. Yorgin et al10 found low erythropoietin levels in 81.6% of their paediatric anemic patients.
The immunosuppressive scheme possible effect was not analysed since 91% of patients received the same regimen of immunosuppression. However, episodes of toxicity, which were not evaluated, could have possibly been an extra factor to anaemia development.
Our study is limited due to its observational nature and the relatively small sample, but it reinforces that the prevalence of post -RT anaemia is high in the paediatric population, that it has a multifactorial nature and, therefore, must be properly assessed and treated.
CONCLUSION
Anaemia has consequences in health (including increasing cardiovascular risk) and quality of life, so it should be well managed. Prevalence of anaemia was relevant in our centre, 67% at 12 months post-RT. Aetiology seems to be multifactorial and although the presence of anaemia pre-RT, GFR reduction, UTI and acute episodes of graft dysfunction were more prevalent in the anaemic group, viral infection, mainly CMV, was the only statistically significant factor at the 12 months analysis.
Prospective and randomized studies are needed to determine the target values of haemoglobin, iron kinetics and the best management of anaemia in these patients. Regular screening of PTA and evaluation of the multiple risk factors associated is recommended.
References
1. Gouva C, Nikopoulos P, Loannidis JP, Siamopoulos KC. Treating anemia early in renal failure patients slows the decline of renal function: a randomized controlled trial. Kidney Int 2004;66(2):753 -760 [ Links ]
2. Al -Uzri A, Yorgin PD, Kling PJ. Anemia in children after transplantation: etiology and the effect of immunosuppressive therapy on erythropoiesis. Pediatr Transplant 2003;7(4):253 -264 [ Links ]
3. Gheit O, Wafa E, Hassan N, Mostafa A, et al. Does posttransplant anemia affect long-term outcome of live -donor kidney transplantation? A single -center experience. Clin Exp Nephrol 2009;13(4):361 -366 [ Links ]
4. Imoagene -Oyedeji AE, Rosas SE, Doyle AM, Goral S, Bloom RD. Posttransplantation anemia at 12 months in kidney recipientes treated with mycophenolate mofetil:risk factors and implications for mortality. J Am Soc Nephrol 2006;17(11):3240 -3247 [ Links ]
5. Rossert J, Fouqueray B, Boffa JJ. Anemia management and the delay of chronic renal failure progression. J Am Soc Nefrol 2003;14(7 Suppl 2):S173 -177 [ Links ]
6. Turkowski-Duhem A, Kamar N, Cointault O, et al. Predictive factors of anemia within the first year post renal transplant. Transplantantion 2005;80(7):903 -909. [ Links ]
7. Karakus S, Kanbay M, Koseoglu HK, Colak T, Haberal M. Causes of anemia in renal transplant recipients.Transplant Proc 2004;36 (1):164 -165. [ Links ]
8. Unal A, Sipahioglu MH, Akcakaya M, et al. An underappreciated problem in renal transplant recipients: anemia. Transplant Proc 2008;40(5):1399 -1403. [ Links ]
9 . National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification and stratification. Am J Kidney Dis 2002;39(2 Suppl 1): S1 -S266 [ Links ]
10. Yorgin PD, Belson A, Sanchez J, et al. Unexpectedly high prevalence of posttransplant anemia in pediatric and young adult renal transplant recipients. Am J Kidney Dis 2002;40(6):1306 -1318. [ Links ]
11. Mitsnefes MM, Subat -Dezulovic M, Khoury PR, Goebel J, Strife CF. Increasing incidence of post-kidney transplant anemia in children. Am J Transplant 2005;5(7):1713 -1718. [ Links ]
12. A. Nathan and Oskis Haematology of Infancy and Childhood. 7thed Philadelphia: Saunders2009 [ Links ]
13. Scigalla P, Bonzel KE, Bulla M, et al. Therapy of renal anemia with recombinant human erythropoietin in children with end-stage renal disease. Contrib Nephrol 1989;76:227 -241 [ Links ]
14. Burke JR. Low -dose subcutaneous recombinant erythropoietin in children with chronic renal failure. Australian and New Zealand Paediatric Nephrology Association. Pediatric Nephrol 1995;9(5):558-561 [ Links ]
15. Einollahi B, Rostami Z, Teimoori M, Beiraghdar F. Late anemia in pediatric kidney transplant recipients: Prevalence and risk factors. Nephro -Urol Mon 2011;3(3):172 -176 [ Links ]
16. Ghafari A, Noori Majelan N. Anemia among long -term renal transplant recipients. Transplant Proc 2008;40(1):186 -188. [ Links ]
17. Miles AM, Markell MS, Daskalakis P, et al. Anemia following renal transplantation: erythropoietin response and iron deficiency. Clin Transplant 1997;11(4):313 -315. [ Links ]
18. Kausman JY, Powell HR, Jones CL. Anemia in pediatric transplant recipients. Pediatr Nephrology 2004;19(5):526 -530 [ Links ]
19. Nampoory MR, Johny KV, Al -Hilali N, Seshadri MS, Kanagasabhapathy AS. Erythropoietin deficiency and relative resistance cause anaemia in post -renal transplant recipients with normal renal function. Nephrol Dial Transplant 1996;11(1):177 -181 [ Links ]
20. Galutira PJ, Del Rio M. Understanding renal posttransplantation anemia in the pediatric population. Pediatr Nephrol 2012;27(7):1079-1085 [ Links ]
21. Abbud Fillho M, Adams PL, Alberu J, et al. A report of the Lisbon Conference on the care of the kidney transplant recipient. Transplantation 2007;83(8 Suppl):S1 -S22. [ Links ]
Dra Sara Ferreira
Pediatrics Department, CHLN Norte -Santa Maria
Avenida Professor Egas Moniz
1649 -035 Lisboa, Portugal
E-mail:
Email: saranf82@hotmail.com
Conflict of interest statement: None declared
Received for publication: 08/01/2014
Accepted in revised form: 19/05/2014














