Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.28 no.2 Lisboa jun. 2014
EDITORIAL
Hepatitis C and dialysis. Are we doing what we should?
Hepatite C e diálise. Estamos a fazer o que devíamos?
Sequeira Andrade
Medical Service. Centro Hospitalar do Médio Tejo. Torres Novas, Portugal
Patients with positive viral serology, mainly those infected with B, C or HI viruses are a matter of concern for nephrologists and other professionals in dialysis units. In this paper I will focus my attention more on hepatitis C patients, which are currently the most prevalent among all seropositive patients under dialysis treatment in Portugal.
Hepatitis C is an issue of great contemporaneity. It became matter of discussion and controversy with frequent news in the media. Some reasons are related to the great results that have been reported with the most recent therapies obtaining cure rates, in some patients, of more than 80%, but other reasons are not so centred on patients interests and have hidden great economic concerns.
There have been huge advances in viral infection knowledge and that is why I ask if we are doing, at the present time, what is correct in terms of evaluation, monitoring and treatment of these patients.
There are many unanswered questions concerning hepatitis C virus (HCV) positive patients: do dialysis patients receive adequate attention as to their clinical situation? That is, are there appropriate study and monitoring protocols for these patients? Do we know which patients we should treat or not? Are the best therapies defined? Are there studies on the use of various drugs in kidney failure? Does each transplantation centre have its own pre-transplantation protocols? Is what is being done what should actually be done?
Attempting to answer these questions, in June 2013, I organized a meeting entitled Hepatitis C and Renal Failure where I brought together renowned nephrologists, gastroenterologists and infectious diseases specialists to discuss those problems. Renal patients, since the early stages of the disease to the phases in which they receive dialysis, haemodialysis, peritoneal dialysis and, even, renal transplantation, pose several clinical problems. They are more susceptible to infection and there must be a definition of the best evaluation protocols or the best therapeutic approaches in the various stages of the disease.
The hepatitis C virus is known since the eighties of the 20th century. The older nephrologists still recall the time when there was no knowledge yet of this virus and hepatic cytolysis was called NANB (non-A, non-B) hepatitis1 and, also, when that entity was the most frequent cause of liver enzymes elevation in haemodialysis patients2. The most sustained documentation came from studies relating to the post-transfusion hepatitis. The first publications that began to point to the viral aetiology are from 1989.
This was the year when Michel Houghton and his collaborators, after several years of study, identified parts of the C virus3 and, subsequently, developed a serological test capable of identifying the virus in infected individuals4.
For some years the haemodialysis units in Portugal reported very high incidence and prevalence of this entity. The registered prevalence nationwide grew till 1993, when 26.5% of dialyzed patients in this country were HCV positive and the highest incidence rate, 9.9%, recorded in 19915. The evolution is summarized in Table 1.
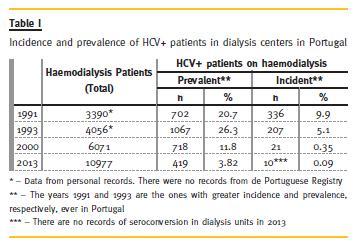
Observing these numbers we can easily say that the measures designed and implemented to address this big problem that invaded the haemodialysis centres had an almost complete success. Here it is worth noting that the daily work and persistence over time of the nursing staff, which was the guarantor of the implementation of those measures, and that those professionals have a great responsibility in these results. An excellent job was performed in terms of epidemiological control but, even so, we had under treatment in Portugal (haemodialysis and peritoneal dialysis), at the end of 2013, about 436 patients with hepatitis C. In fact, these constant control and surveillance measures continue to be of great usefulness and urgency in many countries in the world, mainly in the most populous or less developed6.
On the other hand, at the meeting that I referred earlier, we could realize that the various institutions that have the responsibility of treating haemodialysis patients in Portugal do not have established strategies in terms of screening or treatment of these patients. For example, some perform virus RNA (ribonucleic acid) test in all positive patients but have laid down subsequent actions vis-a-vis the positivity of the exam. Some observed that these patients do not represent increase in expenses when they are compared to negative patients. This clearly suggests that these patients are monitored not differently from the others. We also could not identify co-morbidities, hospital admissions and mortality in this group of patients. The same can be said about the approach by the various renal transplant centres that have, each, their own protocols, and apply them to patients who, for the most part, are enrolled in more than one centre. Or, transplantation centres, simply, do not have protocols at all and depend on the opinions of infectious diseases specialists or gastroenterologists, most of whom have scant experience in the treatment of kidney patients and with whom it is not always easy to contact professionally for discussion of the problems that these patients present.
Why do HCV+ patients have a different approach of the other virus carriers in dialysis centres? Let us see:
The hepatitis B virus (HBV)+ patients receive haemodialysis treatment in separate rooms from the other patients. Their regular assessment, according to the Portuguese Good Practices Manual, does not include analysis for study of their infectivity, or viral replication, or for any consequences of long-term virus liver (chronic active hepatitis, cirrhosis, portal hypertension, HCC). If the patients are positive for the surface antigen they are evaluated for that analysis on an annual basis. Unprotected patients for HBV are subjected to vaccination programmes.
The human immunodeficiency virus (HIV)+ patients, who are already receiving haemodialysis treatment also in private clinics, are already under therapy for HIV and are more or less kept under control with hospital infectious diseases specialists, where the disease is monitored and the therapy provided free of charge.
Patients with HCV+, mostly arriving at private haemodialysis clinics with the information of this positivity and receive dialysis treatment in a so-called geographical isolation within the centre, usually with dedicated dialysis monitor, and also in the vast majority of cases, do not receive any special attention to their clinical situation as compared to other patients. Therefore, the single focus of attention is addressed only to the control of disease transmission within the clinic, that is, the protection is directed to the remaining patients and professionals. But this is not a Portuguese situation exclusively.
An observational study published recently with Outcomes and Practice Patterns Study (DOPPS) data relating to almost 50,000 patients under haemodialysis, in twelve countries during the period between 1996 and 2011, showing a prevalence of HCV + 9.5%, only 1% of patients with antiviral therapy prescription were receiving this medication. In addition, from the HCV+ patients wait-listed for renal transplantation, only 3.7% were receiving treatment7. Is this situation correct or should it be different? Should we monitor the disease and its complications in a more aggressive way? Should we treat, and who should we treat? I mention some arguments for and against the antiviral treatment in the population of patients with IRC.
For:
1. The HCV is associated with increased likelihood of chronic kidney disease (CKD) and the rate of progression to stage 58-10;
2. The HCV infection is a factor of increased global and cardiovascular mortality in HD patients11-13;
3. Hepatitis C treatment can contribute to improve renal and cardiovascular outcomes in diabetics14;
4. Eradication of infection in patients on haemodialysis is recommended15;
5. Liver biopsy may reveal significant changes despite normal liver enzymology over time16;
6. Big advances in therapies became available17,18;
7. Some of the most recent medications have hepatic metabolism19,20,21;
8. Some studies indicate good results, with high rates of sustained virological response (SVR), low rates of abandonment and accessory effects on haemodialysis patients22,23,24;
9. Candidates for renal transplantation should receive treatment of hepatitis C. It should be said that, in spite of the risks, it is possible to treat patients with hepatitis C after renal transplantation without precipitating acute rejection25;
10. Viral replication of HCV has affected renal graft survival in these patients26;
11. However, there is evidence that renal patients with hepatitis C have a significantly worse prognosis of patient and graft in post-transplantation27;
12. Patients who achieve SVR upon finishing this therapy prior to renal transplantation in general do not become positive after transplantation, even with intense immunosuppressive therapy28;
13. It can be considered that treatment is prevention.
Against:
1. The experience with the new medications in renal failure is still very scarce. There are many references about the contraindication for the use in patients with creatinine clearance < 30ml/min29,30;
2. Liver biopsy is considered necessary in some situations to start therapy and this procedure has increased risks in renal patients;
3. Renal patients, compared with those without kidney disease, have less necro-inflammation and liver fibrosis31;
4. Old age and multiple co morbidities.
Therefore, if we want to change this perspective of not considering renal patients with hepatitis C as candidates for treatment (I shall not talk about transplant patients), my proposal would be to trigger the following procedures:
Facing the presence of a patient with HCV positivity, one should attempt to establish, firstly, if the kidney disease can be related, or attributable, to this virus. If so, and if there are no contraindications, therapy should be started32,33.
If the renal disease cannot be related to HCV, then we should try to establish if we are in the presence of a chronic or acute infection:
1. If we are in the presence of an acute infection, there are no doubts about the indication for treatment if there are no contraindications;
2. In case of chronic infection established by the positivity of HCV in two determinations spaced 6 months apart, we should: a. Evaluate the presence of contraindications to therapy, in which situation the therapy should not be done. The main contraindications are:
i. Old age. The majority of the studies excluded patients older than 60, although some included patients up to 70 years old;
ii. Presence of serious co-morbidities. I believe that, here, we can define the term serious as the presence of morbid situations that shorten the patients life expectancy;
b. Others, whose listing you may want to consider before taking any decision, but I may recall situations like uncontrolled anaemia, thrombocytopenia, depression or convulsive disease;
c. In the absence of contraindications, we should carry out the determination of RNA of HCV and determination of viral genotype;
d. If we have a positive result for HCV RNA we have the necessary condition to consider treatment;
e. Request the opinion of a specialist with experience in treating these patients, usually gastroenterology or infectious diseases specialist, with whom we should discuss the indications for treatment, the need to carry out liver biopsy (Fibroscan is a technique not yet validated for renal patients), the patients opinion about the therapy and, eventually, the perspective of renal transplantation34.
Before I finish, I would still like to draw attention to difficulties that the nephrologists in Portugal will find when they search evaluation and treatment of the disease caused by HCV for their chronic renal patients as a result of some constraints that the bundled payment system of dialysis treatment introduced.
This payment scheme limited the scope of action for nephrologists with regard to the evaluation and treatment of co-morbidities of renal patients on dialysis in this country.
In terms of final reflection, I can say that my goal with this text, was to draw attention to the nephrologists who have HCV positive patients under their responsibility, and we all have them, that we should not be pleased with the good work that has been done over the years in terms of epidemiological control in haemodialysis clinics. Leaving these patients without treatment is a situation that will certainly be changed in the near future, considering the enormous developments related to the new medications that are giving us great expectations of cure. Unfortunately, renal patients, and especially those who are already on dialysis, have been out of the scope of the experience which has been accumulated with these new drugs.
This is certainly an area in which one can say that treatment is, or will be, prevention. So, answering the question posed in the title of this article, this is what I think we should do in the near future concerning HCV positive patients with renal failure.
References
1. Breadbear RA.Chronic hepatitis: a review. J R Soc Med 1985;78(5): 391-396 [ Links ]
2. Ribeiro F et al. Hepatite Nao A Nao B numa Unidade de Hemodialise. Rev Port Nefro e Hipert 1990; 4(1); 25-36 [ Links ]
3. Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 1989; 244; 359-362 [ Links ]
4. Alter HJ, Purcell RH, Shih JW, et al. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N Engl J Med 1989;321(22);1494-1500 [ Links ]
5. dos Santos JP, Loureiro A, Cendoroglo Neto M, Pereira BJ. Impact of dialysis room and reuse strategies on the incidence of hepatitis C virus infection in hemodialysis units. Nephrol Dial Transplant 1996;11(10):2017-2022 [ Links ]
6. Khan S, Attaullah S, Ali I, et al. Rising burden of hepatitis C virus in hemodialysis patients. Virology J 2011;8:438 [ Links ]
7. Goodkin DA, Bieber B, Gillespie B, Robinson BM, Jadoul M. Hepatitis C infection is very rarely treated among hemodialysis patients. Am J Nephrol 2013;38(5); 405-412 [ Links ]
8. Chen YC, Lin HY, Li CY, Lee MS, Su YC. A nationwide cohort study suggests that hepatitis C virus infection is associated with increased risk of chronic kidney disease. Kidney Int 2014;85(5):1200-1207 [ Links ]
9. Butt AA, Wang X, Fried LF. HCV Infection and the Incidence of CKD. Am J Kidney Dis 2011;57(3):396-402 [ Links ]
10. Li Wc, Lee YY,Chen IC, Wang SH, Hsiao CT, Loke SS. Age and gender differences in the relationship between hepatitis C infection and all stages of Chronic Kidney Disease. J Viral Hepat 201 3; Dec 5. doi: 10.1111/jvh.12199. [Epub ahead of print] [ Links ]
11. Fabrizi F, Dixit V, Messa P. Impact of hepatitis C on survival in dialysis patients: A link with cardiovascular mortality? J Viral Hepat 2012;19(9):601-607 [ Links ]
12. Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, et al. Hepatitis C virus and death risk in hemodialysis patients. J Am Soc Nephrol 2007;18(5):1584–1593 [ Links ]
13. Fabrizi F, Aghemo A, Messa P. Hepatitis C treatment in patients with kidney disease. Kidney Int 2013;84(5):874–879 [ Links ]
14. Hsu YC, Lin JT, Ho HJ, et al. Antiviral treatment for hepatitis C virus infection Is associated with improved renal and cardiovascular outcomes in diabetic patients. Hepatology 2014;59(4):1293-1302 [ Links ]
15. Khedmat H, Amini M, Ghamar-Chehreh ME1, Agah S. Hepatitis C virus infection in dialysis patients. Saudi J Kidney Dis Transpl 2014;25(1):1-8 [ Links ]
16. Fabrizi F, Dixit V, Messa P, Martin P. Hepatitis C-related liver disease in dialysis patients. Contrib Nephrol 2012;176:42-53 [ Links ]
17. Muir AJ. The rapid evolution of treatment strategies for hepatitis C. Am J Gastroenterol 2014;109(5):628-635 [ Links ]
18. Chao D, Botwin GJ, Morgan TR. Update on recently approved treatments for hepatitis C. Curr Treat Options Gastroenterol 2014;12(2):211–228 [ Links ]
19. Dumortier J, Guillaud O, Gagnieu MC, et al. Anti-viral triple therapy with telaprevir in haemodialysed HCV patients: Is it feasible? J Clin Virol 2013;56(2):146–149 [ Links ]
20. Zhu Y, Chen S.Antiviral treatment of hepatitis C virus infection and factors affecting efficacy. World J Gastroenterol 2013;19(47):8963-8973 [ Links ]
21. de Kanter Drenth JP, Arends JE, et al. Viral hepatitis C therapy: pharmacokinetic and pharmacodynamic considerations. Clin Pharmacokinet 2014;53(5):409–427 [ Links ]
22. Tseng PL, Chen TC, Chien YS, et al. Efficacy and safety of pegylated interferon alfa-2b and ribavirin combination therapy versus pegylated Interferon monotherapy in hemodialysis patients: A comparison of 2 sequentially treated cohorts. Am J Kidney Dis 2013;62(4):789-795 [ Links ]
23. Wang KL, Xing HQ, Zhao H, et al.Efficacy and tolerability of low-dose interferon-α in hemodialysis patients with chronic hepatitis C virus infection. World J Gastroenterol 2014;20(14): 4071-4075. [ Links ]
24. Brennan BJ, Wang K, Blotner S, et al. Safety, tolerability, and pharmacokinetics of ribavirin in hepatitis C virus-infected patients with various degrees of renal impairment. Antimicrob Agents Chemother 2013;57(12)6097–6105 [ Links ]
25. Sanai FM, Mousa D, Al-Mdani A, et al. Safety and efficacy of peginterferon-alpha2a plus ribavirin treatment in renal transplant recipients with chronic hepatitis C. J Hepatol 2013;58(6):1096–1103 [ Links ]
26. European Association for Study of Liver..EASL Clinical Practice Guidelines: Management of hepatitis C virus infection. J Hepatol 2014;60(2):392–420 [ Links ]
27. Fabrizi F, Martin P, Dixit V, Messa P. Meta-analysis of observational studies: hepatitis C and survival after renal transplant. J Viral Hepat 2014;21(5):314–324 [ Links ]
28. Weclawiak H, Kamar N, Ould-Mohamed A, Cardeau-Desangles I, Izopet J, Rostaing L. Treatment of chronic hepatitis C virus infection in dialysis atients: an update. Hepat Res Treat 2010; Article ID 267412, 6 pages [ Links ]
29. AASLD Guidelines, Recommendations for Testing,Managing, and Treating Hepatitis C – revised march 2014. Downloaded from www.hcvguidelines.org on 06/16/2014 [ Links ]
30. Virlogeux V, Pradat P, Bailly F, et al. Boceprevir and telaprevir-based triple therapy for chronic hepatitis C: virological efficacy and impact on kidney function and model for end-stage liver disease score. J Viral Hepat 2014; doi: 10.1111/jvh.12237 [ Links ]
31. Trevizoli JE, de Paula Menezes R, Ribeiro Velasco LF, al. Hepatitis C is less aggressive in hemodialysis patients than in nonuremic patients. Clin J Am Soc Nephrol 2008;3(5):1385-1390
32. Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO clinical practice guidelines for the prevention, diagnosis,evaluation, and treatment of Hepatitis C in chronic kidney disease. Kidney Int 2008;(Suppl 109): S1–S99 [ Links ]
33. Hayat A, Mitwalli A. Hepatitis C and kidney disease. Hepat Res Treat V2010; doi:10.1155/2010/534327 [ Links ]
34. Covic A, Abramowicz D, Bruchfeld A, et al. Endorsement of the Kidney Disease Improving Global Outcomes (KDIGO) hepatitis C guidelines: a European Renal Best Practice (ERBP) position statement. Nephrol Dial Transplant 2009;243):719–727 [ Links ]
Dr. Sequeira Andrade
Medical Area, Nephrology Service, Centro Hospitalar do Medio Tejo
Avenida Xanana Gusmao
2350-399 Torres Novas, Portugal
Email: jcsandrade@gmail.com
Conflict of interest statement: None declared.
Received for publication: 06/06/2014
Accepted: 18/06/2014














