Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.27 no.4 Lisboa dez. 2013
REVIEW ARTICLE
Whats new in hepatorenal syndrome? An updated review for the nephrologist
O que há de novo no Síndrome hepatorrenal? Uma revisão actualizada para o nefrologista
Rita Magrico, Ana Mateus, Aura Ramos
Department of Nephrology, Hospital Garcia de Orta. Almada, Portugal.
ABSTRACT
In advanced cirrhosis, increased levels of vasodilators and impaired cardiac compensatory response decrease effective arterial blood volume, causing vasoconstriction of renal arteries and kidney failure in up to 40% of patients after 5 years of follow-up. Hepatorenal syndrome (HRS) diagnostic criteria are: cirrhosis with ascites; serum creatinine (SCr) > 1.5 mg/dL (with no improvement 2 days after diuretic withdrawal and albumin administration). Shock, nephrotoxics and acute parenchymal kidney disease must be excluded. The HRS is classified in: type 1, defined by a 100% increase in SCr to > 2.5 mg/dL in < 2 weeks, and type 2, with a slower and milder decrease in kidney function. Type 3 HRS is an emerging concept, referring to HRS in patients with coexistent kidney disease. Left untreated, average survival of type 1 HRS is 2 weeks whereas in type 2 it is 6 months. Treatment of HRS lies on reversal of the hepatic disease or liver transplantation (combined liver-kidney transplant may be appropriate for patients who have been on renal replacement therapy (RRT) for more than 8 weeks). However, with todays available therapy, there may be reversibility of HRS without liver transplant. Type 1 HRS is treated with vasoconstrictors (mainly terlipressin; noradrenalin may be an alternative in patients in intensive care units) and albumin. Reversal of HRS occurs in about half of patients. If SCr does not decrease and patients have classic indications for dialysis, RRT can be used as a second-line treatment until liver recovery or transplant. MARS (molecular readsorbent recirculating systems) and Prometheus systems should be considered experimental. Type 2 HRS treatment is based on repeated large-volume paracentesis and albumin administration. If ineffective, vasconstrictors are used. Since renal impairment is mild, RRT is not indicated. If liver recovery/transplant are unfeasible, patients treatment should avoid futilities.
Key words: Dialysis; hepatorenal syndrome; kidney failure; liver cirrhosis; terlipressin.
RESUMO
Na cirrose avançada, os vasodilatadores associados a uma resposta cardiaca inadequada diminuem a volemia, causando vasoconstrição das artérias renais e insuficiência renal em ate 40% destes doentes apos 5 anos de seguimento. Critérios diagnósticos do Síndrome Hepatorrenal (SHR): cirrose com ascite; creatinina sérica >1.5 mg/dL (persistente 2 dias apos suspensão de diuréticos e albumina); exclusão de choque, nefrotoxicos e doença renal parenquimatosa aguda. Classificação do SHR: tipo 1 (duplicação da creatinina inicial para >2.5 mg/dL em <2 semanas) e tipo 2 (agravamento mais lento e ligeiro da função renal). Recentemente, descreve-se ainda o SHR tipo 3, em doentes com SHR e patologia renal simultâneos. Sem tratamento, a sobrevida no SHR tipo 1 e aproximadamente 2 semanas e no SHR tipo 2 seis meses. O tratamento do SHR baseia-se na reversão da doença hepática de base / no transplante hepático (transplantes de fígado-rim reservam-se para doentes em dialise ha mais de 8 semanas). No entanto, com a terapêutica actualmente disponível, pode haver reversão do SHR sem transplante hepático. O SHR tipo 1 trata-se com vasoconstritores (principalmente terlipressina; se doente em Unidade de Cuidados Intensivos, a noradrenalina pode ser uma alternativa) e albumina. Espera-se reversão do SHR em aproximadamente metade dos doentes. Se a creatinina não diminuir e o doente tiver indicações clássicas para dialise, esta pode ser utilizada ate a recuperação hepatica / ao transplante. Os sistemas MARS (Molecular Adsorbent Recirculating System) e o Prometheus devem ser considerados experimentais. O tratamento do SHR tipo 2 consiste em paracenteses evacuadoras periodicas com albumina. Se ineficazes, podem-se usar vasoconstritores. Como a disfunção renal e ligeira, a dialise nao esta indicada. O tratamento dos doentes em que a recuperação hepática / o transplante não são possíveis deve evitar futilidades.
Palavras chave: Cirrose hepática; dialise; insuficiência renal; síndrome hepatorrenal; terlipressina.
INTRODUCTION
In advanced cirrhosis, portal hypertension causes severe vasodilation of the splanchnic arteries, leading to a decrease in effective arterial blood volume and arterial pressure. This leads to an intense stimulation of the renin-angiotensin and sympathetic nervous systems, which cause vasoconstriction of the renal arteries and kidney failure1,2. The high levels of plasma rennin activity, plasma aldosterone concentration and plasma norepinephrine would also be expected to cause a hyperdynamic circulation, with an increase in heart rate, ventricular contractility, and cardiac output in order to compensate for hypotension. However, whereas cardiac output can increase in early stages of cirrhosis, studies in HRS patients show no increase in heart rate and, actually, a decrease in cardiac output3. Therefore, three key mechanisms seem to contribute to HRS: splanchnic vasodilatation with hypotension and reduced renal perfusion, renal artery vasoconstriction and cardiac inability to compensate, so that kidney failure seems to result from haemodynamic imbalance, with a preserved tubular function. The kidney failure in HRS is considered a functional defect because there is reversibility of the condition with vasoconstriction of the splanchnic circulation or with liver transplant. Although there is a common misconception that the kidneys are histologically normal, a relatively specific but subtle and reversible renal lesion has been described–glomerular tubular reflux4.
In advanced cirrhosis, the vasodilation of the splanchnic arteries is caused by: 1) greater production and activity of vasodilators such as (the most important) nitric oxide and others, such as endogenous cannabinoids and carbon monoxide5-8; 2) proinflammatory cytokines with vasodilatation activity produced in response to bacterial translocation from the intestinal lumen to mesenteric lymph nodes9,10; 3) neoangiogenesis in mesenteric arteries and impaired response to vasoconstrictors11. The vasodilators spread along the systemic circulation leading to a global decrease in systemic vascular resistance.
These mechanisms constitute the Classical Peripheral Arterial Vasodilation Hypothesis.
As stated earlier, impaired inothropic and chronotropic cardiac responses are also important, and led to a revision of this traditional hypothesis. The pathogenesis of the impaired cardiac response in HRS is largely unknown. Contributing factors to reduced cardiac output may be: 1) organic – attenuated systolic and diastolic responses to stress stimuli resulting from the cirrhotic cardiomyopathy, common in patients with HRS; 2) functional – related to a decrease in venous return. Supporting this theory are the facts that: i) HRS occurs in the setting of a decrease in cardiopulmonary pressures, which is compatible with a fall in cardiac preload; ii) intravenous albumin associated with vasoconstrictors and TIPS are included in the treatment of HRS, and both these strategies increase venous return. The impairment in chronotropic cardiac function is probably related to a down regulation of b-adrenergic receptors secondary to the chronic stimulation of the sympathetic nervous system3.
EPIDEMIOLOGY
The incidence of HRS is highly variable depending on the studies (10 to 40% after 5 years of follow up of a population of patients with cirrhosis and ascites).
In a prospective study of 229 nonazotemic patients with cirrhosis and ascites the hepatorenal syndrome developed in 18 and 39 percent at one and five years, respectively12. Patients with hyponatremia and a high plasma renin activity were at highest risk. These signs of neurohumoral activation presumably reflected a more severe decline in effective perfusion13,14.
The hepatorenal syndrome characteristically occurs in patients with advanced hepatic disease and in the presence of portal hypertension (hepatic cirrhosis) and / or hepatic insufficiency (severe alcoholic hepatitis, hepatic metastases or fulminant hepatitis from any cause)13,15,16.
Although hepatorenal syndrome can be seen in most forms of severe hepatic disease, patients with primary biliary cirrhosis appear relatively protected17, possibly due in part to the natriuretic and renal vasodilator actions of retained bile salts.
DIAGNOSTIC CRITERIA
To be diagnosed with HRS, patients have to fulfill all the criteria18 presented in Table I, as proposed by the International Ascites Club in 2007. When comparing these criteria with the former ones (published in 1996)5, there was a significant advance in terms of simplification of the diagnosis, hence permitting an earlier, more effective treatment. The more important differences between the old and the new criteria are described below. In the 1996s criteria, there had to be a serum creatinine of > 1.5 mg/dL or a 24-h creatinine clearance of < 40 mL/min.
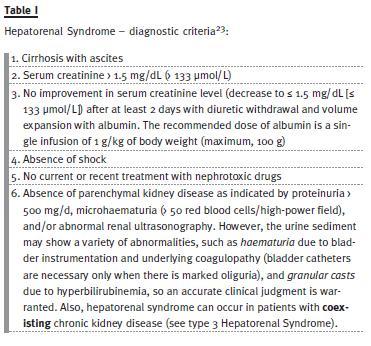
Data showed that 24-h creatinine clearance takes longer to obtain, is more complicated to measure and does not improve diagnostic accuracy. Therefore, the current recommendation is to use serum creatinine. Secondly, in the 1996s diagnostic criteria, HRS diagnosis was made after a non-specified period of diuretic withdrawal and volume expansion with saline. New data clearly favour plasma expansion with albumin rather than with saline. Additionally, new diagnostic criteria refer specifically that the attempt of volume expansion should last no more than 48h to begin treatment of HRS. Also, the 1996s criteria specifically referred that was necessary to exclude gastrointestinal or renal losses of fluid whereas in the new diagnostic criteria the clinician focuses on whether volume expansion improves creatinine. Thirdly, in the 1996s diagnostic criteria, an acute infection actually excluded HRS. Nowadays we know that only sepsis must be excluded, as the most common trigger for the development of type 1 HRS is actually bacterial infection, particularly spontaneous bacterial peritonitis (SBP). This way, treatment can (should) be started without waiting for complete recovery from the infection. Finally, in the 1996s diagnostic criteria, existed additional criteria (low urinary volume, serum and urinary sodium – U Na < 10mEq/day – and high urine to plasma osmolality ratio).
Although often present, these parameters are not essential for the diagnosis and so were removed. Looking again at Table I, we realize the efforts made to simplify the diagnostic criteria and start treatment as soon as possible. However, HRS remains a diagnostic of exclusion and a diagnostic marker is still lacking.
CLINICAL TYPES OF HRS18
Type 1
Rapidly progressive decrease in kidney function: 100% increase in serum creatinine to a final value > 2.5 mg/dL (> 221 μmol/L) in < 2 weeks. The clinical presentation is usually that of acute kidney failure.
Type 2
Stable or slowly progressive decrease in kidney function that does not meet the criteria of type 1. The clinical picture is that of ascites refractory to diuretic therapy.
Type 319,20Some authors have singled out a type 3 hepatorenal syndrome, in which there is coexistent kidney disease and hepatorenal syndrome. Although at first sight this may seem odd (because in the diagnostic criteria one has to exclude the presence of parenchymal renal disease), one has to consider that, for example, diabetics with diabetic nephropathy and non-alcoholic fatty liver disease may develop hepatorenal syndrome.
Other systemic diseases may similarly affect both the liver and the kidney. In fact, a recent study found that 85% of end-stage cirrhotics had pre-existing intrinsic renal disease on renal biopsy21,22. In some cases renal biopsy may be necessary for diagnosis and for selection of patients for combined liver-kidney transplant.
DIFFERENTIAL DIAGNOSIS (Table II)

The hepatorenal syndrome is a diagnosis of exclusion, and other diseases need to be considered5,18,13,15, namely:
– Severe sepsis
– Other causes of severe kidney failure that can arise in patients with advanced cirrhosis: Drug-induced nephrotoxicity (NSAIDs, aminoglycosides, radiological contrasts, others). Pre-renal failure due to volume depletion (from diarrhoea, vomiting, increased dieresis due to use of diuretics or not; other causes of hypovolaemia). Acute Tubular Necrosis (ATN): ATN is usually suspected from the history and from the often rapid rise in the serum creatinine, which contrasts to the usually gradual rise in hepatorenal syndrome. Some of the traditional laboratory methods used to distinguish pre-renal disease from ATN (such as the urinalysis or the fractional excretion of sodium) may not be helpful in patients with hepatic disease. Glomerulonephritis in patients with hepatitis B or C; Immunoglobulin A nephropathy (mainly combined with alcoholic cirrhosis); others Distinguishing the hepatorenal syndrome from these other disorders is clinically important because of the marked difference in prognosis and the urgent need to begin treatment directed to hepatorenal syndrome.CLINICAL PRESENTATION
Type 1 HRS24:
Severe and progressive kidney failure. However, due to the marked reduction in creatinine production among such patients, the serum creatinine may increase by as little as 0.1 mg/dL (9 micromol/L) per day, with intermittent periods of stabilization or even slight improve ment24. Severe circulatory dysfunction (mean arterial pressure usually is 70 mm Hg) and very low systemic vascular resistance. Severe liver disease, with jaundice, coagulopathy, low albumin levels, hepatic encephalopathy, poor nutritional status, and large ascites and oedema. Urine volume usually is not extremely reduced and some patients may have normal urine volumes, with markedly lower output being observed only within a few days from death25,26.Type 2 HRS2:
Moderately severe kidney failure (serum creatinine levels of ~ 2.0 mg/dL) of functional origin that remains stable for variable periods. Ascites, usually resistant to diuretic therapy (because of the combined influence of profound sodium retention, reduced GFR, and markedly increased levels of aldosterone and norepinephrine). Dilutional hyponatremia. Some patients with type 2 HRS develop type 1 HRS, which may arise spontaneously or as a result of some complication, usually a bacterial infection.Type 3 HRS:
Development of HRS in a patient with coexistent kidney disease.ADDITIONAL DIAGNOSTIC WORKUP
Liver disease may be associated with near normal values for both the BUN (due to decreased urea production) and the serum creatinine (due to muscle wasting) despite a relatively large reduction in GFR27,28. The presence of kidney disease in this setting can be documented by a reduction in creatinine clearance, but significant overestimation of GFR can still occur28,29-31. Because of the problems with changes in creatinine production and secretion, other endogenous compounds have been evaluated in an effort to provide a more accurate estimation of GFR, including cystatin C and urinary biomarkers such as neutrophil gelatinase-associated lipocalin (NGAL).
Cystatin C: It has been proposed that cystatin C-based equations would be more accurate in patients with cirrhosis when compared with creatinine clearance.
For example, in a study in liver chirrhotic patients32, a cystatin C-based formulae (developed by Larsson and Hoek) showed significantly lower bias and higher precision than the creatinine-based formulae (Cockroft&Gault or MDRD) for GFR estimation. However, both creatinine and Cystatin C-based equations overestimated the true GFR by 105-154%. Therefore, even in populations in which cystatin C would be expected to outperform creatinine based GFR calculations, cystatin C-based equations are not totally accurate33.
Also, even if cystatin C proved to be more accurate for the assessment of GFR than serum creatinine in cirrhotics, whether measurement of cystatin C levels would improve patient care is at present unknown, so cystatin C is not at the moment routinely performed in these patients.
Urinary biomarkers, such as neutrophil gelatinaseassociated lipocalin (NGAL), tend to be lower in pre-renal azotaemia and hepatorenal syndrome than in acute tubular necrosis (ATN), but there is considerable overlap between these conditions34,35.
PRECIPITATING FACTORS2:
– None (in some patients)
– After effective arterial blood volume is decreased by:
Bacterial infections and, in particular, spontaneous bacterial peritonitis. Approximately onethird of patients with spontaneous bacterial peritonitis develop HRS and are treated simultaneously for both disorders. Of these patients, about one-third experience reversal of HRS when the infection is resolved. However, the remainder develop either stable (type 2) or progressive HRS (type 1). Patients who develop type 1 HRS as a result of spontaneous bacterial peritonitis have a dismal outcome, with almost 100% hospital mortality if not treated appropriately.Infections other than spontaneous bacterial peritonitis also may cause HRS, but its frequency and severity usually are lower than that of patients with spontaneous bacterial peritonitis.
Gastrointestinal bleeding. The development of kidney failure after gastrointestinal bleeding is not very common in patients who have cirrhosis (10%) and it is almost fully confined to patients with hypovolaemic shock. In most instances, it is associated with ischaemic hepatitis, which implies that the kidney failure most likely is related to ATN and not HRS.
Large volume paracentesis (> 5 L) in the absence of albumin administration. Largevolume paracentesis without albumin may trigger HRS in 15% or more of cases.Although diuretics have often been mentioned as precipitants of HRS, diuretics do not cause hepatorenal syndrome. Diuretics can, however, cause azotaemia, which improves with the cessation of therapy and fluid repletion, while in the hepatorenal syndrome kidney function typically worsens inexorably, even after diuretics are stopped.
TREATMENT OF HRS
Type 1 HRS
Treatment of type 1 HRS can be divided into three stages, depending on the severity of the hepatic and renal disease. These stages are: treatment of the hepatorenal syndrome itself, treatment of the acute kidney injury and treatment of the advanced hepatic disease. The first stage, treatment of the hepatorenal syndrome itself, includes pharmacological treatment (with intravenous albumin, to expand intravascular volume, together with vasoconstrictors, to reverse splanchnic vasodilatation) and interventional procedures (transjugular intrahepatic portosystemic shunt – TIPS – and peritoneovenous shunt). The second stage, treatment of the acute kidney injury (with renal replacement therapy) is necessary when the pharmacological treatment is ineffective or is still underway in patients who are candidates to kidney transplant or in whom reversal of the hepatic disease is a possibility. The final stage, treatment of the advanced hepatic disease, either by resolving the hepatic insult or by liver transplant, is the only definite treatment (artificial support of the hepatic and renal function may be temporarily required, in which case the MARS and Prometheus techniques may be of use).
Treatment of the hepatorenal syndrome itself Pharmacological treatment
Albumin and vasoconstrictors
Intravenous albumin in association with vasoconstrictors (Table III) improves survival and is currently considered the best therapy for type 1 HRS13,36. This approach is intended to expand intravascular volume and to cause vasoconstriction of the greatly dilated splanchnic arterial bed. This in turn alleviates arterial underfilling, lessens the activation of the endogenous vasoconstrictor systems, and increases kidney perfusion and GFR.

– Administration of albumin: 1 g/kg body weight at day 1 and 2 – maximum 100g – followed by 25-50 g/d.24
– Administration of vasoconstrictor drugs2:
Terlipressin (vasopressin analogue, acts on V1 vasopressin receptors in vascular smooth muscle cells): - 1 mg/4-6 h as IV bolus;- The dose is increased up to a maximum of 2 mg/4-6 h after 3 days if there is no response to therapy, defined by a decrease in serum creatinine > 25% of pre-treatment values.
- Response to therapy is indicated by a marked decrease in the high serum creatinine levels, to less than 1.5 mg/dL (< 133 μmol/L).
Treatment is usually given from 5-15 days (occasionally longer if there is some but not complete improvement of renal function after two weeks).
Noradrenaline or midodrine (α-adrenergic agonists, act on α1-adrenergic receptors in vascular smooth muscle cells):- Norepinephrine (given in an ICU): 0.5-3 mg/h as continuous intravenous infusion aimed at increasing mean arterial pressure by 10 mmHg. Treatment is maintained until serum creatinine decreases to < 1.5 mg/dL (< 133 μmol/L).
- Midodrine (a systemic vasoconstrictor): 7.5 mg orally at eight-hour intervals, increased to a maximum of 15 mg 3id if needed. Treatment with the combination of terlipressin and albumin is associated with reduced mortality and reversal of HRS in 40%-50% of patients37, making this approach the preferred initial therapy (according to the European Association for the Study of the Liver – EASL – 2010 guidelines)36. However, given the fact that some studies38,39 found no difference in efficacy and safety between patients treated with terlipressin plus albumin versus noradrenalin plus albumin, and because terlipressin may be significantly more expensive than noradrenalin, noradrenalin is recommended by some authors24 as first line therapy for patients with HRS who are in a ICU (noradrenalin perfusion is not usually available on the general medical ward). However, in Portugal, this price difference may not be always present so an individual approach is warranted. Response to treatment with terlipressin and albumin is associated with a progressive decrease in serum creatinine concentration, increased urine output, and improvement of hyponatremia.
Factors that predict a response to treatment are an increase in arterial pressure during treatment and low baseline creatinine level. After withdrawal of therapy, HRS recurs in < 15% of patients, and in these cases, a second treatment with terlipressin is usually effective. The incidence of side effects (usually ischaemic) that mandate discontinuation of treatment is ~12%. Terlipressin has been associated with an increase of cardiovascular adverse events37.
Alternatives to terlipressin (especially useful in countries where terlipressin is not available, such as the USA) are noradrenaline and midodrine (low cost; broad availability) plus octreotide (100 to 200 μg subcutaneously 3x/d; or as a continuous intravenous infusion – 50 mcg/hr), both in combination with albumin. Octreotide is a somatostatin analogue, which inhibits the release of endogenous vasodilators, producing splanchnic vasoconstriction. The speed with which effective treatment is achieved appears to be important. Thus, some authors24 prefer continuous infusion of octreotide rather than subcutaneous injection and adjustment of each consecutive midodrine dose (every 8 hours) in order to rapidly achieve an increase in blood pressure instead of changing the dose only 24 hours later. In patients who respond to therapy, midodrine is occasionally used indefinitely to maintain a higher mean arterial pressure (or until there is resolution of liver injury or liver transplantation is performed). Also, midodrine may be effective in many patients who recover from type 1 hepatorenal syndrome who continue to have refractory ascites.
Patients treated with vasoconstrictors should be followed up carefully throughout treatment for early detection of side effects, particularly cardiovascular events and pulmonary oedema.
Alternative therapies
Acutely lowering renal sympathetic tone and renal vascular resistance in the early stages of hepatorenal syndrome by the intravenous administration of the sympatholytic agent, clonidine, can raise the GFR by as much as 25 percent56. However, this benefit does not appear to be sustained with chronic oral therapy, despite a persistent reduction in sympathetic activity57.
Interventional procedures
TIPS
A recent study showed that vasoconstrictor therapy followed by stent placement (TIPS – transjugular intrahepatic portosystemic shunt) was effective in a limited number of patients with type 1 HRS2,45,46.
This approach is sometimes successful in highly selected patients, who fail to respond to vasoconstrictors and albumin and who are well enough to undergo the procedure. However, this procedure is associated with numerous complications (high incidence of encephalopathy, among others) and, because of the need for intravenous contrast, it may cause acute kidney injury. For this reason, some experts prefer dialysis as a first option (continuous renal replacement therapy) for patients whose serum creatinine remains above 1.5 mg/dL despite medical therapy. Overall, the available results suggest that TIPS should be considered only as a last resort in selected patients24. More studies are required to establish the value of TIPS placement in the treatment of HRS.
Peritoneovenous shunt
Peritoneovenous shunts drain peritoneal fluid from the peritoneum into the internal jugular vein, reinfusing ascites into the vascular space. It is now rarely used because of an appreciable rate of complications and lack of evidence that peritoneovenous shunting prolongs patient survival24.
Goal of therapy
The goal of medical therapy or TIPS in patients with hepatorenal syndrome is reversal of the acute kidney injury (decrease in the high serum creatinine levels, to least < 1.5 mg/dL, < 133 μmol/L). In addition, when patients are treated with norepinephrine, terlipressin, or midodrine plus octreotide, an immediate goal of therapy is to raise the mean arterial pressure by approximately 10 to 15 mmHg24. The magnitude of the increase in mean arterial pressure induced by these vasoconstrictors appears to be significantly associated with the magnitude of the decrease in serum creatinine58. As an example, in a systematic review of 501 patients with hepatorenal syndrome from 21 studies, a 9 mmHg increase in mean arterial pressure predicted a 1 mg/dL (88.4 micromol/L) decrease in serum creatinine. If a patient has no improvement in renal function after two weeks, therapy with these drugs can be considered futile24.
T reatment of the acute kidney injury
– when vasoconstrictors are ineffective Renal replacement therapy
RRT is not considered the first-line treatment for patients with type 1 HRS because it does not correct the underlying pathogenesis2. RRT should be started when patients with type 1 HRS are unresponsive to vasoconstrictors and when there are signs of uraemia, volume overload, severe metabolic acidosis, or hyperkalaemia.
However, not all patients are candidates for dialysis. Dialysis is useful as a bridge to liver transplantation or until there is liver recovery2. Bridging patients to liver transplantation includes patients either waiting for a transplant or being evaluated for liver transplantation. In patients who develop a need for RRT but who are not expected to recover liver function / to receive a liver transplant, long-term RRT is usually not indicated / should be withhold40 except as a trial to see if renal function will return42.
Haemodialysis is frequently difficult to perform in patients with hepatorenal syndrome since decompensated hepatic function is associated with haemodynamic instability, thrombocytopenia and coagulopathy.
Survival with RRT is poor with only 30–60% of patients surviving to liver transplant42. Some success has been accomplished with continuous renal replacement (CRRT) modalities41, which have potential advantages such as improved cardiovascular stability, more gradual correction of hyponatraemia (necessary to avoid central pontine myelinolysis), less fluctuation in intracranial pressure and removal of inflammatory cytokines, such as TNF-α and IL-6.
Studies, however, do not show superiority of CRRT when compared with conventional intermittent RRT.
However, all trials available are non-randomized with populations considered to be non-comparable to one another. Therefore, the decision of which modality to choose continues to be based on the clinical characteristics of the patient as dictated by haemodynamic stability and severity of illness42.
T reatment of the advanced hepatic disease
– looking for a definitive treatment
Although with todays available therapy there may be reversibility of HRS without liver transplant18, the definitive treatment of hepatorenal syndrome is improvement of liver function (for example, by recovery of alcoholic hepatitis, treatment of decompensated hepatitis B with effective antiviral therapy or recovery from acute hepatic failure), or liver transplantation.
In patients in which recovery of liver function may be expected or who are candidates for liver transplantation, temporary artificial support of the liver (and renal) function may be considered, using either the MARS (molecular readsorbent recirculating system) or FPSA (fractionated plasma separation and adsorption – the Prometheus system).
MARS and Prometheus
The MARS system is designed to remove albuminbound toxins (including vasodilators) by albumin dialysis as well as providing standard continuous renal replacement therapy (CRRT). The albumin dialysate is then regenerated utilizing an anion exchange resin and active charcoal adsorption. MARS has been utilized in treatment of HRS and was shown to be superior to CRRT in terms of patient survival, improved haemodynamics and urine output42. However, no large-scale trial has been carried out and more recent studies (6 patients) in patients with type 1 HRS not responding to vasoconstrictor therapy found no improvement following MARS therapy in terms of systemic haemodynamics42. Also, in a French study with thirty-two patients with type 1 hepatorenal syndrome, MARS therapy improved renal function in only very few patients with type 1 HRS43.
Fractionated plasma separation and adsorption (FPSA) is a method of albumin dialysis that is integrated into an extracorporeal liver support device (Prometheus). In the HELIOS trial, a randomized-controlled European multicenter trial of FPSA therapy, a total of 145 patients with acute-on-chronic liver failure were either treated with standard medical treatment and FPSA eight to 11 times over 21 days or with standard medical treatment alone. There was no statistically significant difference in the overall survival.
However, significant survival benefit was observed under FPSA therapy in a predefined subgroup of patients with type 1 hepatorenal syndrome44.
In conclusion, although MARS and Prometheus systems may be used to bridge patients to liver transplant, controlled studies are needed. Until then, these therapies should be considered experimental42.
Liver transplant
Liver transplant is the first choice of treatment for patients with cirrhosis and type 1 HRS because of their low survival expectancy2. Therefore, patients who are candidates for liver transplant should be referred immediately to transplant centres.
Because kidney failure is reversible after liver transplant, combined liver-kidney transplant is generally considered appropriate only for patients who have been on RRT for more than 8 weeks who have a low likelihood of recovery of kidney function47,48.
However, one must consider that the exact duration of pre-liver transplant kidney dysfunction or dialysis that is amenable to recovery is not known. Retrospective studies from single centres have shown the importance of the duration of > 12 weeks of SCr ≥ 1.5 mg/dL and dialysis ≤ 4 weeks pre-transplant on post-transplant renal outcomes49-52. The duration of pre-transplant dialysis may be variable according to the physician/centre considered, so these results must be viewed with caution. The decision for combined liver-kidney versus liver transplant alone should be undertaken with consideration of duration of HRS, AKI and CKD and risk factors for progression of CKD present at the time of liver transplant such as hypertension, diabetes and obesity40. In summary, the 8th international consensus conference of the Acute Dialysis Quality Initiative (ADQI) Group40, suggest liver transplantation alone for candidates with type-1 HRS for less than four weeks and simultaneous liver-kidney (SLK) for those at risk for nonrecovery of renal function (2D).
Provision of intra-operative continuous RRT during liver transplant may be indicated to help control volume and electrolytes42. Use of vasoconstrictors before liver transplant with the aim of performing transplant on patients with normal or near-normal kidney function remains an open question, because studies are scarce and include a small number of patients. However, excellent survival has been reported with the 2 approaches (transplant-without treating HRS or treat HRS before transplant) 53,54,55.
Patients who are not candidates for transplant or who have important comorbid conditionsDecisions about the management of patients who are not candidates for transplant or who have important comorbid conditions should be made on an individual basis2. In these patients, therapy with vasoconstrictors should be individualized and RRT probably be reserved for particular cases (potentially reversible chronic liver diseases – alcoholic hepatitis, acute-on-chronic liver failure, etc. – with no important associated comorbid conditions) in order to avoid futilities.
Type 2 HRS
– Usually managed as outpatients
– Spironolactone and other potassium-sparing diuretics should generally be avoided because of the risk of hyperkalaemia, whereas loop diuretics, such as furosemide, usually lack efficacy.
However, diuretics can be given to patients without adverse reactions to diuretics who have a sodium excretion under diuretic treatment of > 30 mEq/d2.
– Treatment of ascites is based on repeated largevolume paracentesis and albumin administration (8 g/1 L of ascites removed)36,59.
– More studies are required to more fully understand the role that vasoconstrictors plus albumin and TIPS may have in treating type 2 HRS. Some algorithms propose the use of vasoconstrictors in patients with type 2 HRS who are candidates for liver transplant and in whom there is a rise in serum creatinine2.
– Renal replacement therapy is not indicated in the management of patients with type 2 HRS because of the lack of a severe decrease in kidney function.
Prevention of HRS2,24,36:
– Administration of IV albumin to all patients with cirrhosis and spontaneous bacterial peritonitis (1.5 g/kg body weight at diagnosis and 1 g/kg 48 hours later) – as proposed in the guidelines of the European Association for the Study of the Liver (EASL) – reduces kidney impairment and improves survival.
– Long-term oral administration of norfloxacin (400 mg/d) in patients with ascitic fluid protein < 15 g/L and associated decreased liver and/or kidney function (bilirubin > 3 mg/dL [> 51.3 μmol/L], Child-Pugh score > 10, serum sodium < 130 mEq/L [< 130 mmol/L], and/or serum creatinine > 1.2 mg/dL [> 106.1μmol/L]) reduces the risk of HRS and improves survival. These effects probably are related to prevention of bacterial translocation, suppression of pro-inflammatory cytokines, and improvement in circulatory function.
– One report suggested that the reduction in intrahepatic pressure induced by transjugular intrahepatic portosystemic shunt (TIPS) placement may prevent the development of the hepatorenal syndrome. This retrospective study evaluated 204 patients with variceal bleeding who were treated with either a portasystemic shunt or sclerotherapy (or other non-shunt modalities) 60. Portasystemic shunting was associated with a lower incidence of ascites (15 versus 73%) and hepatorenal syndrome (4 versus 21%), a higher incidence of encephalopathy, and no difference in overall patient survival60.
Prognosis
– If untreated, median survival of type 1 HRS is only 2 weeks. In type 2, average median survival is 6 months. The best hope for reversal of the renal failure is an improvement in hepatic function due to partial resolution of the primary disease or to successful liver transplantation.
The rate of recovery of kidney function following recovery of liver failure is uncertain. However, a substantial proportion of patients who have progressed to dialysis and survive to receive a liver transplant do recover kidney function61.
References
1. Gines P, Cardenas A, Schrier RW. Liver disease and the kidney. In: Schrier RW, ed. Diseases of the Kidney and Urinary Tract. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2007;2179-2205 [ Links ]
2. Fagundes C, Gines P. Hepatorenal syndrome: A severe, but treatable, cause of kidney failure in cirrhosis. Am J Kidney Dis 2012;59(6):874-885 [ Links ]
3. Arroyo V, Fernandez J, Gines P. Pathogenesis and treatment of hepatorenal syndrome. Semin Liver Dis 2008;28(1):81–95 [ Links ]
4. Kanel GC, Peters RL. Glomerular tubular reflux- a morphologic renal lesion associated with the hepatorenal syndrome. Hepatology 1984;4(2):242-246 [ Links ]
5. Arroyo V, Gines P, Gerbes AL, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology 1996;23(1):164-176 [ Links ]
6. Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodes J. Peripheral arterial vasodilatation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology 1988;8(5):1151-1157 [ Links ]
7. Martin PY, Gines P, Schrier RW. Nitric oxide as a mediator of hemodynamic abnormalities and sodium and water retention in cirrhosis. N Engl J Med 1998;339(8):533-541 [ Links ]
8. Ros J, Claria J, To-Figueras J, t al. Endogenous cannabinoids: a new system involved in the homeostasis of arterial pressure in experimental cirrhosis in the rat. Gastroenterology 2002;122(1):85-93
9. Wiest R, Das S, Cadelina G, Garcia-Tsao G, Milstien S, Groszmann RJ. Bacterial translocation in cirrhotic rats stimulates eNOS-derived NO production and impairs mesenteric vascular contractility. J Clin Invest 1999;104(9):1223-1233 [ Links ]
10. Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology 2005;41(3): 422-433 [ Links ]
11. Iwakiri Y. Endothelial dysfunction in the regulation of cirrhosis and portal hypertension. Liver Int 2012;32(2):199-213 [ Links ]
12. Gines A, Escorsell A, Gines P, t al. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology 1993;105(1):229-236
13. Gines P, Schrier RW. Renal failure in cirrhosis. N Engl J Med 2009;361(13):1279-1290 [ Links ]
14. Fernandez-Seara J, Prieto J, Quiroga J, t al. Systemic and regional hemodynamics in patients with liver cirrhosis and ascites with and without functional renal failure. Gastroenterology 1989;97(5):1304-1312
15. Gines P, Guevara M, Arroyo V, Rodes J. Hepatorenal syndrome. Lancet 2003;362(9398): 1819-1827 [ Links ]
16. Wadei HM, Mai ML, Ahsan N, Gonwa TA. Hepatorenal syndrome: pathophysiology and management. Clin J Am Soc Nephrol 2006;1(5):1066-1079 [ Links ]
17. Better OS. Renal and cardiovascular dysfunction in liver disease. Kidney Int 1986; 29(2):598-607 [ Links ]
18. Salerno F, Gerbes A, Gines P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut 2007;56(9):1310-1318 [ Links ]
19. Hartleb M, Gutkowski K. Kidneys in chronic liver diseases. World J Gastroenterol 2012;18(24): 3035-3049 [ Links ]
20. Rajekar H, Chawla Y. Terlipressin in hepatorenal syndrome: Evidence for present indications. J Gastroenterol Hepatol 2011;26 Suppl 1:109–114 [ Links ]
21. McGuire BM, Julian BA, Bynon JS, t al. Brief communication: Glomerulonephritis in patients with hepatitis C cirrhosis undergoing liver transplantation. Ann Intern Med 2006;144(10): 735–741
22. McGuire B, Julian B, Novak J et al. High prevalence of glomerulonephritis in patients with end-stage HCV-induced cirrhosis. Hepatology 2007; 46: 474A [ Links ]
23. Wong F, Nadim MK, Kellum JA, t al. Working Party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut 2011;60(5): 702-709
24. Bruce A Runyon, Richard H Sterns, John P Forman, Hepatorenal syndrome in: UptoDate, Topic 2308 Version 9.0, viewed at Jun 11 2013 [ Links ]
25. Baldus WP, Feichter RN, Summerskill WH, The kidney in cirrhosis. I. Clinical and biochemical features of azotemia in hepatic failure. Ann Intern Med 1964;60:353-365 [ Links ]
26. Esrailian E, Pantangco ER, Kyulo NL, Hu KQ, Runyon BA. Octreotide/Midodrine therapy significantly improves renal function and 30-day survival in patients with type 1 hepatorenal syndrome. Dig Dis Sci 2007;52(3):742-748 [ Links ]
27. Papadakis MA, Arieff AI. Unpredictability of clinical evaluation of renal function in cirrhosis. Prospective study. Am J Med 1987;82(5):945-952 [ Links ]
28. Sherman DS, Fish DN, Teitelbaum I. Assessing renal function in cirrhotic patients: problems and pitfalls. Am J Kidney Dis 2003;41(2):269-278 [ Links ]
29. Proulx NL, Akbari A, Garg AX, Rostom A, Jaffey J, Clark HD. Measured creatinine clearance from timed urine collections substantially overestimates glomerular filtration rate in patients with liver cirrhosis: a systematic review and individual patient meta-analysis. Nephrol Dial Transplant 2005;20(8):1617-1622 [ Links ]
30. Skluzacek PA, Szewc RG, Nolan CR 3rd, Riley DJ, Lee S, Pergola PE. Prediction of GFR in liver transplant candidates. Am J Kidney Dis 2003;42(6):1169-1176 [ Links ]
31. Knight EL, Verhave JC, Spiegelman D, t al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int 2004;65(4):1416-1421
32. Poge U, Gerhardt T, Stoffel-Wagner B, Klehr HU, Sauerbruch T, Woitas RP. Calculation of glomerular filtration rate based on cystatin C in cirrhotic patients. Nephrol Dial Transplant 2006;21(3):660-664 [ Links ]
33. Stevens LA, Padala S, Levey AS. Advances in glomerular filtration rate-estimating equations. Curr Opin Nephrol Hypertens 2010;19(3):298-307 [ Links ]
34. Verna EC, Brown RS, Farrand E, t al. Urinary neutrophil gelatinase-associated lipocalin predicts mortality and identifies acute kidney injury in cirrhosis. Dig Dis Sci 2012;57(9):2362-2370
35. Fagundes C, Pepin MN, Guevara M, et al. Urinary neutrophil gelatinase-associated lipocalin as biomarker in the differential diagnosis of impairment of kidney function in cirrhosis. J Hepatol 2012;57(2):267-273 [ Links ]
36. Gines P, Angeli P, Lenz K, t al. For the European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol 2010;53(3):397-417
37. Gluud LL, Christensen K, Christensen E, Krag A. Terlipressin for hepatorenal syndrome. Cochrane Database Syst Rev 2012;9:CD005162 [ Links ]
38. Singh V, Ghosh S, Singh B, t al. Noradrenaline vs. terlipressin in the treatment of hepatorenal syndrome: a randomized study. J Hepatol 2012;56(6):1293-1298
39. Alessandria C, Ottobrelli A, Debernardi-Venon W, et al. Noradrenalin vs terlipressin in patients with hepatorenal syndrome: a prospective, randomized, unblinded, pilot study. J Hepatol 2007;47(4):499-505 [ Links ]
40. Nadim MK, Kellum JA, Davenport A, t al. Hepatorenal syndrome: the 8th International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group, Crit Care 2012:16(1):R23
41. Epstein M. Hepatorenal syndrome: emerging perspectives. Semin Nephrol 1997;17(6):563-575 [ Links ]
42. Gonwa TA, Wadei HM. The challenges of providing renal replacement therapy in decompensated liver cirrhosis, Blood Purif 2012;33/1-3):144–148 [ Links ]
43. Lavayssiere L, Kallab S, Cardeau-Desangles I, et al. Impact of molecular adsorbent recirculating system on renal recovery in type 1 hepatorenal syndrome in patients with chronic liver failure. J Gastroenterol Hepatol 2013;28(6):1019-1024 [ Links ]
44. Rifai K. Fractionated plasma separation and adsorption: current practice and future options. Liver Int 2011;31(Suppl 3):13–15 [ Links ]
45. Schroeder ET, Anderson GH Jr, Smulyan H. Effects of a portacaval or peritoneovenous shunt on renin in the hepatorenal syndrome. Kidney Int 1979;15(1):54-61 [ Links ]
46. Rossle M, Gerbes AL. TIPS for the treatment of refractory ascites, hepatorenal syndrome and hepatic hydrothorax: a critical update. Gut 2010;59(7):988-1000 [ Links ]
47. Eason JD, Gonwa TA, Davis CL, Sung RS, Gerber D, Bloom RD. Proceedings of Consensus Conference on Simultaneous Liver Kidney Transplantation (SLK). Am J Transplant 2008;8(11):2243-2251 [ Links ]
48. Charlton MR, Wall WJ, Ojo AO, et al. Report of the first international liver transplantation society expert panel consensus conference on renal insufficiency in liver transplantation. Liver Transpl 2009;15(11)(suppl 2):S1-S34 [ Links ]
49. Campbell MS, Kotlyar DS, Brensinger CM, et al. Renal function after orthotopic liver transplantation is predicted by duration of pretransplantation creatinine elevation. Liver Transpl 2005;11(9):1048-1055 [ Links ]
50. Bahirwani R, Campbell MS, Siropaides T, t al. Transplantation: impact of pretransplant renal insufficiency. Liver Transpl 2008;14(5):665-671
51. Ruiz R, Kunitake H, Wilkinson AH, t al. Long-term analysis of combined liver and kidney transplantation at a single center. Arch Surg 2006;141(8):735-741
52. Ruiz R, Barri YM, Jennings LW, t al. Hepatorenal syndrome: a proposal for kidney after liver transplantation (KALT). Liver Transpl 2007;13(6):838-843
53. Rice JP, Skagen C, Said A. Liver transplantation outcomes for patients with hepatorenal syndrome treated with pretransplant vasoconstrictors and albumin. Transplantation 2011;91(10):1141-1147 [ Links ]
54. Boyer TD, Sanyal AJ, Garcia-Tsao G, t al. Impact of liver transplantation for the survival of patients treated for hepatorenal syndrome type 1. Liver Transpl 2011;17(11):1328-1332
55. Restuccia T, Ortega R, Guevara M, t al. Effects of treatment of hepatorenal syndrome before transplantation on posttransplantation outcome. A case-control study. J Hepatol 2004;40(1):140-146
56. Esler M, Dudley F, Jennings G, t al. Increased sympathetic nervous activity and the effects of its inhibition with clonidine in alcoholic cirrhosis. Ann Intern Med 1992;116(6):446-455
57. Roulot D, Moreau R, Gaudin C, t al. Long-term sympathetic and hemodynamic responses to clonidine in patients with cirrhosis and ascites. Gastroenterology 1992;102 (4 Pt 1):1309-1318
58. Velez JC, Nietert PJ. Therapeutic response to vasoconstrictors in hepatorenal syndrome parallels increase in mean arterial pressure: a pooled analysis of clinical trials. Am J Kidney Dis 2011;58(6):928-938 [ Links ]
59. Salerno F, Guevara M, Bernardi M, t al. Refractory ascites: pathogenesis, definition and therapy of a severe complication in patients with cirrhosis. Liver Int 2010;30(7):937-947
60. Castells A, Salo J, Planas R, t al. Impact of shunt surgery for variceal bleeding in the natural history of ascites in cirrhosis: a retrospective study. Hepatology 1994;20(3):584-591
61. Marik PE, Wood K, Starzl TE. The course of type 1 hepato-renal syndrome post liver transplantation. Nephrol Dial Transplant 2006;21(2):478-482 [ Links ]
Dra Rita Magrico
Department of Nephrology, Hospital Garcia de Orta
Av. Prof. Torrado da Silva – Pragal
2805-267, Almada Portugal
E-mail: rita.magrico@yahoo.com
Conflict of interest statement: None declared.
Received for publication: 14/06/2013 Accepted in revised form: 18/11/2013














