Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.27 no.2 Lisboa abr. 2013
Renal replacement therapy in critically ill patients – what modality should we choose?
Terapêutica substitutiva da função renal no doente crítico – que modalidade escolher?
Ana Carina Ferreira
Department of Nephrology, Centro Hospitalar de Lisboa Central – Hospital de Curry Cabral, Lisboa, Portugal
ABSTRACT
Acute kidney injury is a common complication in the intensive care unit. Mortality in critically ill patients requiring dialysis is unacceptably high, despite significant advances in the care of the critically ill with AKI.
The discussion whether continuous or intermittent renal replacement therapy is the preferred modality of choice in these patients has decades and the two main factors for decision are: the availability and experience with a specific treatment, and the haemodynamic status of the patient. Multiple studies have tried to establish the best treatment option in terms of patient and renal survival for critically ill patients requiring dialysis. In this revision I will try to summarize the available evidence on this topic.
Key-words: Acute kidney injury; continuous renal replacement therapy; intensive care unit; intermittent dialysis.
RESUMO
A lesão renal aguda é uma complicação comum nas unidades de cuidados intensivos. A mortalidade do doente crítico que requer diálise é extremamente elevada, apesar dos avanços significativos dos cuidados prestados a estes doentes. Há várias décadas que se discute o tipo de modalidade dialítica a oferecer a estes doentes (continua ou intermitente) e os principais fatores que pesam na decisão clínica são os meios e a experiência do centro, bem como a condição clínica do doente. Vários estudos tentaram estabelecer a melhor abordagem ao doente crítico com lesão renal aguda e necessidade dialítica, em termos de sobrevida do doente e recuperação renal. Nesta revisão tentarei resumir as evidências disponíveis sobre este tema.
Palavras-chave: Lesão renal aguda; diálise intermitente; técnicas contínuas; unidade de cuidados intensivos.
INTRODUCTION
Acute kidney injury (AKI), characterized by sudden impairment of kidney function, is a common complication in critically ill patients, occurring in 30% to 60%1-3 and leading to decreased survival. The Acute Dialysis Quality Initiative Workgroup, in 2004, developed a set of criteria for defining and classifying AKI, i.e., the RIFLE classification, in which AKI is classified according to its severity4. In September 2005, a new classification was proposed by the Acute Kidney Injury Network (AKIN), the AKIN classification, introducing small though important modifications to RIFLE5. Lopes JA6 published a good review of the definition of AKI in the latest issue of this Journal.
Acute kidney injury severe enough to require renal replacement therapy (RRT) involves roughly 5% of intensive care unit (ICU) patients1,2 and is associated with increased mortality that can reach 60% to 80%7,8. So, mortality rates remain unacceptably high despite significant advances in the care of the critically ill with AKI9. The decision of beginning dialysis in a critically ill patient can lead to disagreement among clinicians, since the therapeutic aims, the optimal timing for initiation, the dosing and the modality remain uncertain.
Conventional indications for RRT include hyperkalaemia or severe metabolic acidosis and fluid overload, not controlled by medical treatment, or uremic symptoms and treatment of poisoning with a few agents10, in order to preserve the life of the patient, allowing for organ recovery. Worth mentioning that fluid overload has recently been claimed as a major outcome determinant of critically ill patients with AKI, and it seems that, besides associated with mortality, fluid overload may also be associated with a decreased likelihood of renal recovery11. This suggests the need to decrease fluid administration in patients with AKI or to target negative fluid balance during RRT in these patients12. Dialysis is also used in some non-renal indications based on the presumed elimination of inflammatory mediators, on the removal of fluid or elimination of other endogenous toxic solutes13, for instance in sepsis, acute respiratory distress syndrome and congestive heart failure. These indications are limited and not established, as currently we have insufficient data to recommend that14,15. In fact, a very recent randomized trial involving patients hospitalized for acute congestive heart failure, worsened renal function and persistent congestion, showed that a stepped pharmacologic therapy algorithm is superior to a strategy of ultrafiltration for the preservation of renal function, with fewer adverse events16.
Timing of initiation of RRT in critically ill patients with AKI is an unresolved issue, and some studies and a recent meta-analysis evidence for early institution of RRT, since this approach may lead to benefit on survival17,18. Nevertheless, overall design and quality of studies comparing the two strategies (early versus late) is low18. Further research on this is necessary.
The delivery of dialysis dose is another controversial problem, and two randomized controlled studies (RENAL and ATN), compared an intensive treatment strategy with a more conventional renal support19,20 (Table I). Both showed that more intensive RRT dose did not improve patient survival, recovery of kidney function, or duration of RRT and, therefore, the conventional dosing is the recommended (effluent flow rate target of 20-25 ml/Kg/h).
In this article I will describe briefly the different RRT modalities used in critically ill patients, and review the relevant randomized trials that compare these modalities with the aim of summarizing the available evidence on this topic.
RRT IN CRITICALLY ILL PATIENTS
When we decide to dialyse a critically ill patient in an ICU, the first thing to do is to choose the modality to use: intermittent haemodialysis (IHD) – conventional, 3 times a week / 4h; or hybrid, also known as slow low-efficient dialysis (SLED); – continuous renal replacement therapy (CRRT) – continuous venovenous (CVV) RRT; peritoneal dialysis (PD); or slow continuous ultrafiltration (SCUF).
The discussion whether CRRT or IHD is the preferred modality of choice for RRT in the ICU has decades, and it appears that most nephrologists preferred IHD, whereas intensivists preferred CRRT7, since the introduction of this modality in the clinical practice in the late 1970s/early 1980s was to compensate the inadequacies of the IHD in the treatment of critically ill patients with multiple -organ failure21.
Peritoneal dialysis, as a CRRT, could also be used, but there is lack of evidence in AKI adults patients, in view of the fact that the studies are generally confine to paediatrics, reports in adults are mainly uncontrolled observations, and, contrary to what occurs in developing countries, it is infrequently used in AKI in high-income countries. Currently, indications may include bleeding diathesis, haemodynamic instability and difficulty in obtaining vascular access22.
Nevertheless, complications with this technique in an ICU are high and include protein loss, peritonitis, ventilator compromise or high glucose levels.
Slow continuous ultrafiltration is used mainly as a dehydrating procedure for fluid removal by filtration and, when solute control is important, it has to be supplemented with either IHD or CRRT.
When selecting the modality, the two main factors are: the availability and experience with a specific treatment, and the patient´s haemodynamic status.
If the patient is haemodynamic stable, we have no doubt, and the patient does conventional IHD. If the patient is unstable, and both modalities are available, we can choose IHD/SLED or CRRT22. Both can provide solute removal by diffusive small-solute transport (haemodialysis), convective small - and medium – solute transport (haemofiltration – HF) or by the combination of diffusive and convective solute transport (haemodiafiltration – HDF), using low – or high -flux dialyzers for haemodialysis and high -flux dialyzers for haemofiltration and haemodiafiltration.
SLED
Slow low-efficient dialysis is a hybrid haemodialysis that has emerged as a viable alternative to conventional IHD and to CRRT in the treatment of AKI patients, and employs characteristics of both modalities23, combining advantages of CRRT with the practicality of IHD. First described in 198823, SLED uses the conventional IHD equipment, but with a slower clearance of solutes and volume [blood flow (Qb) of 100 -300 ml/min and dialysate rates (Qd) of 200-500 ml/min; Ultrafiltration 150 ml/h], for extended periods of time (6-12h/day). Comparing to conventional IHD, it offers less small solutes disequilibrium, provides effective control of azotaemia and confers better haemodynamic tolerance to ultrafiltration.
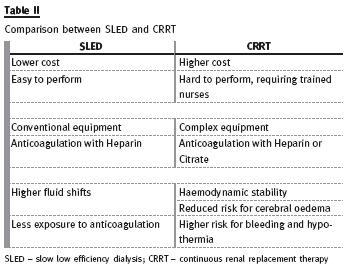
Its popularity is gained because it is easy, safe, convenient, effective and less expensive than CRRT, with similar outcomes to other modalities23. It is likely that this modality offers an additional advantage for conventional IHD, but this remain to be proven (Table II).
CRRT
The patient with severe haemodynamic instability often cannot tolerate conventional intermitente treatments or even SLED. Continuous renal replacement therapy is generally well tolerated, because it has slower fluid removal, resulting in more haemodynamic stability, fewer episodes of renal ischaemia, better control of fluid balance, avoidance of fluid shifts, and reduced risk of cerebral oedema (Qb 100 -200ml/min; dialysate rates of 1000 -2000 ml/min; effluent volume 20 -30 ml/Kg/h)21,22. Continuous therapies perform slow correction of AKI leading to a steady-state condition very similar to that provided by native kidneys21, but frequently delivered dose is different (and lower 10% to 15%) from prescribed dose, most likely due to treatment downtime19,20.
Nevertheless, there are some disadvantages: CRRT uses different and complex equipment, requiring trained nurses for carrying out the technique, leading to higher costs. Besides, it requires immobilization of the patient, there is a risk of hypothermia, and the need for prolonged anticoagulation may increase bleeding risk. Regional heparin/protamine and citrate are safe, but underused. Citrate should be used with caution in patients in shock and in patients with severe liver failure.
Its use requires an established protocol with instructions for infusion of citrate and calcium, composition of the dialysate/replacement fluid, and intensive metabolic monitoring, including acid -base status, sodium and total/ionized calcium levels22 (Table II).
RRT IN ICU PATIENTS – WHAT DO STUDIES TELL US
Multiple observational and retrospective studies have tried to establish the best treatment option for critically ill patients requiring dialysis. In the 1990s, retrospective non -randomized studies reported that patients who received CRRT (using synthetic membranes) had a better survival rate than patients who received IHD (with cuprophan membranes)24 -26; on the other hand, observational or randomized studies with selection bias showed that patients who received IHD had a lower mortality rate, doubtless because CRRT was chosen for the worse cases27,28.
Since 2000 until the present, several randomized controlled studies have been published with the same thematic: to try to find the best patient care and renal survival benefits when using one technique or another.
I chose four such studies (Table III) attempting to answer the question: what is the best modality to dialyze a critically ill patient with AKI? The first study was published, in 2004, by Augustine and co-workers29. They reviewed all cases of patients with AKI requiring dialyses that had been admitted to the ICU of the Cleveland Clinic, between November 1995 and January 1999. Exclusion criteria were previous dialysis treatment/kidney transplant, permanence in ICU less than 48h, or inability to obtain informed consent.
Of the 105 patients admitted to ICU with inclusion criteria, 80 were randomized, according to the Cleveland Clinic Foundation severity score: 40 patients to CRRT (CVVHD; Qb 200 ml/min) and 40 patients to IHD (Qb 300 ml/min, Qd 500 ml/min, 3 times a week, and variable treatment time). Overall hospital mortality was high and similar in both groups (CRRT 67.5%; IHD 70%, p > 0.05); urine output significantly declined in both groups, without differences between modalities.
Total fluid balances were markedly different between groups during the first 3 days on dialysis, the median cumulative total fluid balance was negative ( - 4005 ml) for the CRRT group, and was positive (+1539 ml) for the IHD group ( p < 0.001). This difference was probably related with haemodynamic instability in the former group. Moreover 40% of IHD patients required more vasopressors during dialysis ( p = 0.005). Also changes and the mean decline in MAP on dialysis were different between the two groups: there was a significant decrease in MAP for the IHD patients from the baseline within 72h of dialysis therapy, otherwise MAP remained unchanged on CRRT therapy; the mean decline was high also in IHD patients ( p = 0.047 for the difference between groups). The authors concluded that mortality in critically ill patients requiring dialysis treatment is high, but the modality choice had no impact on renal or patient survival. Even though, CRRT patients achieved greater volume removal preserving haemodynamic stability.
The second, the HEMODIAFE Study30, was performed in 21 French intensive care units, from October 1999 to March 2003, with AKI and RRT patients with multiple-organ dysfunction syndrome (MODS). Exclusion criteria were obstructive/vascular AKI, pregnancy, age (< 18 years), chronic kidney disease (CKD), use of ACEi after admission, haemorrhagic diathesis, SAPS II< 37, moribund state/survival expectancy of less than 8 days. The authors randomized 359 patients: 184 for IHD (Qb ≥ 250 ml/min; Qd 500 ml/min; sodium 150 mmol/L; dialysate temperature 35 °C, treatment time at least 4h); 175 for CRRT (CVVHDF predilutional; Qb ≥120 ml/min; Qd ≥ 500 ml/h; ultrafiltration flow of 1000ml/h). Switches between treatments were authorized and six patients were switched from IHD to CRRT (haemodynamic instability, technical problems, unauthorized switch) and 31 from CRRT to IHD [14 for a planned reason (resolved MODS) and 17 due to technical problems, bleeding or risk of bleeding and lack of efficiency].
There was no difference in 60-day survival (32% IHD; 33% CRRT), length of stay in hospital/ICU, or recovery of renal function. Adverse events were similar between the two modalities, except for hypothermia that occurred less often in IHD modality.
Of note, mean volume loss during each treatment did not differ between the groups, and there were no differences in the incidence of severe arterial hypotension between the two groups. The authors concluded that virtually all patients could be treated with IHD provided that measures were implemented to prevent haemodynamic instability.
The third study was published in Nephrology Dialysis Transplantation, in 2009, by the ivestigators of the Stuivenberg Hospital Acute Renal Failure (SHARF) project31, a predictive model for hospital mortality in patients admitted to ICU. The SHARF score was developed in SHARF 1 and SHARF 2 studies32, and validated in SHARF 3 study33. The present study is the SHARF 4, which was designed to compare hospital and renal survival in patients with AKI requiring dialysis. All adult AKI patients with a serum creatinine ≥ 2 mg/dl, admitted to 9
Belgian ICU, between April 2001 and March 2004, were registered; randomization occurred when RRT was necessary, and patients were stratified in three classes of disease severity, according to the SHARF score (< 30; 30 -60; > 60) and within each stratum, patients were randomized to daily IHD (Qb 100 -300 ml/min; Qd 300 -500 ml/min, 4 -6h), or CRRT (post-dilution CVVHF; Qb 100 -250 ml/min; ultrafiltration rate of 1 -2L/h). Exclusion criteria were age (< 18 years) and CKD. Of the 1303 patients enrolled, 650 required RRT and 316 were randomized: 144 patients for IHD and 172 for CRRT. Of note, 344 patients were excluded by either non -medical reasons (54%) or medical reasons (in 37% – coagulations disturbances or haemodynamic instability perhaps because the authors believed it was incompatible with the use of IHD). Overall mortality was 60.1% (62.5% for IHD; 58.1% for CRRT, p=0.430) and no difference in mortality between both treatment options could be observed within each of the three SHARF classes. The authors concluded that, in this controlled randomized trial with stratification according to disease severity, mortality rates, the length of hospital and ICU stay, and renal function at hospital discharge were comparable for both treatment options.
The last study I chose was published in 201034. In continuity with the previous study using the same database, the authors searched the patients with AKI who survived to hospitalization, at 2 years after discharge. The survivors of the SHARF 4 were used for the follow -up to investigate long-term mortality, renal function, co -morbidity and quality of life.
Of the 1303 patients enrolled, only 595 survived hospitalization after AKI. The first conclusion was that AKI is a very severe disease. Of the 595, 182 died within 2 years after discharge (non-survivors), and 413 (survivors) were eligible for further investigation. Comparing survivors at 2 years with non-survivors, the latter were older (p < 0.001), proportionally more male (p = 0.036), and mean creatinine clearance at discharge was significantly lower (p = 0.030). Binary logistic regression analysis noticed only age and gender as independent predictors of long-term mortality.
No differences between survivors and non–survivors were observed in disease severity, length of ICU or hospital stay, late ICU admission, type or cause of AKI, neither treatment modality (conservative treatment, IHD, CRRT). The authors confirmed the poor prognosis of AKI, with a hospital mortality rate of 50.7%, and showed that sepsis, ventilation, late ICU admission were not predictive for long -term mortality, as they are in predicting mortality in ICU and hospital.
The authors also confirmed that the modality of RRT has no influence in long -term outcome of AKI. Of the survivors, only 204 received a home visit (142 were lost to follow-up and 67 did not consent): a considerable part of survivors stayed in need of chronic RRT (21 patients) and survivors had important co–morbidities (peripheral vascular disease, peptic ulcer disease, diabetes and myocardial infarction).
THE MESSAGE LEARNED
These studies suggest that dialysis modality does not influence mortality. Despite the theoretical benefit of CRRT, no study has clearly proved it. But we must bear in mind that none of the studies was perfect: the studies were small (only two included more than 300 patients and were prospective, randomized, and multicentred in design30,31), the exclusion of the very haemodynamic unstable31; absence of a comparison of the delivered dose of dialysis with both modalities29-32; absence of comparison between SLED and CRRT or even conventional IHD and SLED; comparison between continuous haemodiafiltration versus standard intermittent haemodialysis30-32, were some of the biases encountered.
Given the lack of evidence, some researchers have suggested that CRRT is not cost-effective when comparing to IHD35.
However there are some exceptions where CRRT must be used22,36:
1. In critically ill AKI patients with severe haemodynamic instability, as Augustine and co–workers showed29. SLED may also be tolerated in situations where CRRT is not available22.
2. In patients with acute brain injury or increased intracranial pressure or generalized brain oedema, as occurs in trauma or hepatorenal syndrome.
IHD is associated with fluid shifts and can exacerbate dialysis disequilibrium, resulting in increase of cerebral oedema and intracranial pressure. This situation can be avoided with CRRT22,36-39.
So, in conclusion, no RRT is ideal for all patients with AKI. Also, CRRT and IHD should be seen as complementary therapies in AKI patients22,36. Both modalities are possible in the majority of AKI critically ill patients, considering, however, that there are particular situations that benefit more with CRRT. Clinicians should look for the patient instead of the disease, when choosing RRT.
References
1. de Mendonça A, Vicente JL, Suter PM, et al. Acute renal failure in the ICU: risk factors and outcome evaluated by the SOFA score. Intensive Care Med 2000;26(7):915-921 [ Links ]
2. Bagshaw SM, George C, Dinu I, Bellomo R. A multi -centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol Dial Transplant 2008;23(4):1203 -1210 [ Links ]
3 - Singbartl K, Kellum JA. AKI in the ICU: definition, epidemiology, risk stratification, and outcomes. Kidney Int 2012;81(9):819 -825 [ Links ]
4. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P and the ADQI workgroup. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004;8(4):R204 -212 [ Links ]
5. Metha RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11(2):R31 doi:10.1186/cc5713 [ Links ]
6 - Lopes JA. Acute kidney injury: definition and epidemiology. Port J Nephrol Hypert 2013;27:15 -22 [ Links ]
7. Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 2005;294(7):813-818 [ Links ]
8. Ympa YP, Sakr Y, Reinhart K, Vicent JL. Has mortality from acute renal failure decreased? A systematic review of the literature. Am J Med 2005;118(8):827-832 [ Links ]
9. Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol 2008;3(3):844-861 [ Links ]
10. Lameire N, Van Biesen W, Vanholder R. Acute renal failure. Lancet 2005;365:417-430 [ Links ]
11. Bouchard J, Soroko SB, Chertow GM, et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int 2009;76(4):422-427 [ Links ]
12. Ricci Z, Ronco C. New insights in acute kidney failure in the critically ill. Swiss Med Wkly 2012;142:w13662 [ Links ]
13. Schetz M. Non-renal indications for continuous renal replacement therapy. Kidney Int Suppl 1999;56:S88-S94 [ Links ]
14. Briglia AE. The current state of nonuremic applications for extracorporeal blood purification. Semin Dial 2005;18(5):380 -390 [ Links ]
15. Fox JG, Simpson K, Traynor JP. Should non -oliguric acute renal failure be treated with renal replacement therapy? Port J Nephrol Hypert 2007;21:255-259 [ Links ]
16. Bart BA, Goldsmith SR, Lee KL, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012;367(24):2296 -2304 [ Links ]
17. Liu KD, Himmelfarb J, Paganini E, et al. Timing of initiation of dialysis in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol 2006;1(5):915-919 [ Links ]
18. Karvellas CJ, Farhat MR, Sajjad I, et al. A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: a systematic review and meta-analysis. Crit Care 2011;15(1):R72 [ Links ]
19. RENAL Replacement Therapy Study Investigators, Bellomo R, Cass A, Cole L, et al. Intensity of continuous renal -replacement therapy in critically ill patients. N Engl J Med 2009;361(17):1627 -1638 [ Links ]
20. VA/NIH Acute Renal Failure Trial Network, Palevsky PM, Zhang JH, OConnor TZ, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 2008;359(1):7 -20
21. Ronco C, Cruz D, Bellomo R. Continuous renal replacement in critical illness. Contrib Nephrol 2007;156:309-319 [ Links ]
22. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Kidney Inter Suppl 2012;2:1-138 [ Links ]
23. Tolwani AJ, Wheeler TS, Wille KM. Sustained low-efficiency dialysis. Contrib Nephrol 2007;156:320 -324 [ Links ]
24. Bellomo R, Farmer M, Parkin G, Wright C, Boyce N. Severe acute renal failure: a comparison of acute continuous hemodiafiltration and conventional dialytic therapy. Nephron 1995;71(1):59 -64 [ Links ]
25. van Bommel E, Bouvy ND, So KL, et al. Acute dialytic support for the critically ill: intermittent hemodialysis versus continuous arteriovenous hemodiafiltration. Am J Nephrol 1995;15(3):192 -200 [ Links ]
26. Kierdorf H. Continuous versus intermittent treatment: clinical results in acute renal failure. Contrib Nephrol 1991;93:1-12 [ Links ]
27. Swartz RD, Messana JM, Orzol S, Port FK. Comparing continuous hemofiltration with hemodialysis in patients with severe acute renal failure. Am J Kidney Dis 1999;34(3):424-432 [ Links ]
28. Mehta RL, McDonald B, Gabbai FB, et al. A randomized clinical trial of continuous versus intermittent dialysis for acute renal failure. Kidney Int 2001;60(3):1154-1163 [ Links ]
29. Augustine JJ, Sandy D, Seifert TH, Paganini EP. A randomized controlled trial comparing intermittent with continuous dialysis in patients with ARF. Am J Kidney Dis 2004;44(6):1000-1007 [ Links ]
30. Vinsonneau C, Camus C, Combes A, et al. Continuous venovenous haemodiafiltration versus intermittent haemodialysis for acute renal failure in patients with multiple-organ dysfunction syndrome: a multicentre randomised trial. Lancet 2006;368:379 -385 [ Links ]
31. Lins RL, Elseviers MM, Van der Niepen P, et al. Intermittent versus continuous renal replacement therapy for acute kidney injury patients admitted to the intensive care unit: results of a randomized clinical trial. Nephrol Dial Transplant 2009;24(2):512-518 [ Links ]
32. Lins RL, Elseviers M, Daelemans R, et al. Prognostic value of a new scoring system for hospital mortality in acute renal failure. Clin Nephrol 2000;53(1):10-17 [ Links ]
33. Lins RL, Elseviers MM, Daelemans R, et al. Re -evaluation and modification of the Stuivenberg Hospital Acute Renal Failure (SHARF) scoring system for the prognosis of acute renal failure: an independent multicentre, prospective study. Nephrol Dial Transplant 2004;19(9):2282-2288 [ Links ]
34. Van Berendoncks AM, Elseviers MM, Lins RL, for the SHARF Study Group. Outcome of acute kidney injury with different treatment options: long-term follow-up. Clin J Am Soc Nephrol 2010;5(10):1755-1762 [ Links ]
35. Prowle JR, Bellomo R. Continuous renal replacement therapy: recent advances and future research. Nat Rev Nephrol 2010;6(9):521 -529 [ Links ]
36. Palevsky PM, Liu KD, Brophy PD, et al. KDOQI US Commentary on the 2012 KDIGO Clinical Practice Guideline for Acute Kidney Injury. Am J Kidney Dis 2013;61(5):649-672 [ Links ]
37. Davenport A, Will EJ, Losowsky MS, Swindells S. Continuous arteriovenous haemofiltration in patients with hepatic encephalopathy and renal failure. Br Med J 1987; 295:1028 [ Links ]
38. Davenport A, Will EJ, Davison AM. Early changes in intracranial pressure during haemofiltration treatment in patients with grade 4 hepatic encephalopathy and acute oliguric renal failure. Nephrol Dial Transplant 1990;5(3):192-198 [ Links ]
39. Davenport A. Continuous renal replacement therapies in patients with acute neurological injury. Semin Dial 2009;22(2):165 -168 [ Links ]
Ana Carina Ferreira
Department of Nephrology, Centro Hospitalar de Lisboa Central
– Hospital de Curry Cabral,
Rua da Beneficência nº 8
1069 -166 Lisboa, Portugal
E-mail: a.carina.costa.ferreira@gmail.com
Conflict of interest statement. None declared.
Received for publication: 30/04/2013
Accepted: 06/05/2013














