Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.27 no.1 Lisboa jan. 2013
Unusual solutions for catheter placement in haemodialysis patients
Alternativas na colocação de cateteres em hemodiálise
Fernando Caeiro1, Dulce Carvalho1, Sandra Brum2, Fernando Nolasco1
1 Nephrology Department,Centro Hospitalar de Lisboa Central. Lisboa, Portugal.
2 Nephrology Department,Hospital do Santo Espirito. Angra do Heroísmo, Portugal.
ABSTRACT
Background:Although vascular access is essential for adequate haemodialysis delivery, the systematic use of a patient's venous patrimony may eventually lead to exhaustion of suitable sites for placement of a new vascular access.
Case Report: We present two cases of such patients. In the first one we inserted a 55cm catheter through the left external iliac vein, and a 40cm translumbar catheter was placed in the second one. Both interventions were performed percutaneously under radiological guidance. Both patients were anticoagulated after the procedure.
Conclusion: Unusual sites for haemodialysis catheter placement may be life saving in selected situations and offer safe and viable alternatives for adequate haemodialysis delivery.
Key-Words: Catheters, haemodialysis, vascular access failure.
RESUMO
Introdução: Apesar dos acessos vasculares serem essenciais para uma hemodiálise eficiente, o uso sistemático do património venoso dos doentes pode eventualmente levar a uma exaustão dos locais disponíveis para a colocação de um novo acesso vascular.
Caso Clínico: Apresentamos dois destes casos. No primeiro, foi inserido um cateter de 55cm através da veia ilíaca externa esquerda, no segundo colocou-se um cateter de 40cm translombar. Ambas as intervenções foram efectuadas por via percutânea e sob controlo radiológico. Os doentes foram anticoagulados após o procedimento.
Conclusão: O uso de locais alternativos para a colocação de cateteres de hemodiálise, pode em casos selecionados oferecer uma alternativa viável e segura para estes doentes.
Palavras-Chave: Cateteres,falência de acesso vascular, hemodiálise.
INTRODUCTION
Effective vascular access is essential for an adequate delivery of haemodialysis (HD). However, access-maturation failure and thrombosis associated with the progressive use of a patients venous patrimony may eventually lead to situations where creation of a permanent access is no longer possible.
This is an ever-growing concern, as there is increasing prevalence of elderly and diabetic patients among the dialysis population, which has resulted in higher rates of access maturation failure1.
Jugular veins are the preferred site for catheter implantation since they are associated with the best blood flows and have a lower infection record.
Repeated catheterisation of central veins is, however, a well-known cause of venous damage and stenosis2, which, when associated with catheter infection and thrombosis, leads to progressive loss of adequate sites for catheter placement. In these situations alternative sites for catheter placement must be sought.
Options such as the subclavian and femoral veins are frequent alternatives, but after thrombosis of these sites external iliac veins and inferior vena cava may be viable options to offer efficient dialysis to our patients.
In this case report, we describe two such patients in whom, after exhaustion of the usual sites for access placement, an unusual access site was used, permitting adequate dialysis delivery.
CASE REPORT 1
We present a 77-year-old Caucasian male patient with coronary heart disease, cerebrovascular disease, prostate cancer under hormonal therapy and diverticulosis, on haemodialysis, since 2005, due to hypertensive nephroangiosclerosis. He was switched to peritoneal dialysis, in 2010, as a consequenc e of, exhaustion of the vascular access sites. The patient developed a pleuro-peritoneal leak that relapsed in spite of an attempt at pleurodesis, even after a three-month switch to HD. Treatment was performed through a 24cm long catheter placed in the right femoral vein under radiological guidance, since there was thrombosis of the right common iliac vein and no other central veins were available. Recirculation data were not available; however, dialysis efficiency was low, with an eKt/V of 0.8.
He was admitted to our unit, in July 2011, due to catheter dysfunction, despite several administrations of alteplase, anaemia secondary to haemorrhoidal bleeding (haemoglobin 6.9 g/dL) and hyperkalaemia (K 7.4 mEq/L). A peritoneal dialysis (PD) catheter was placed by mini-laparotomy technique for emergent dialysis and a Doppler analysis of the central veins was performed, revealing a patent left external iliac vein, with thrombosis of the left common femoral vein. A 55cm catheter (Split Cath, Medcomp, Harleysville, PA, USA) was placed percutaneously in the left external iliac vein up to the right atrium (Fig. 1).
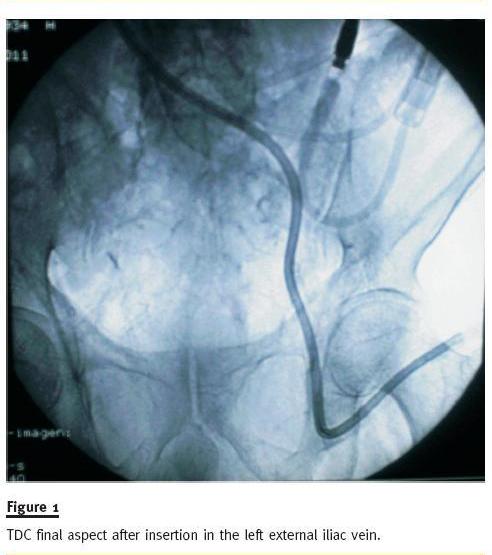
The external iliac vein was visualized and punctured under echographic guidance with an 18G introducer needle. The catheter guidewire was placed through the introducer needle, and sequential dilation of the catheter tract was performed. The metal guidewire was then replaced by a 0.035 hydrophilic catheter (Merit H2O Diagnostic, Merit Medical, South Jordan, UT, USA), and the catheter was placed in situ through it. Afterwards, the PD catheter was carefully sterilized using iodine solution and hypertonic saline and was placed in a subcutaneous position similar to the one described in the Moncrief-Popovich technique3 in case new HD catheter dysfunction should arise in the future (Fig 2).
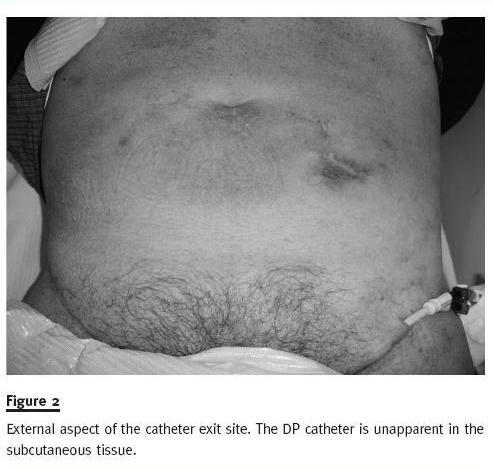
The patient has been placed under anticoagulation and, after 12 months of follow-up, has blood flow of 300-350 ml/min, requiring infrequent administrations of alteplase, after stoppage of the anticoagulation.
CASE REPORT 2
We present a 69-year-old Caucasian female with chronic renal disease due to analgesic nephropathy, on dialysis since 1995. She underwent priority renal transplantation due to vascular access site exhaustion, in 1999. In 2008, she started PD owing to graft dysfunction but, after three months, had to abandon the technique due to a massive pleuroperitoneal leak. After multiple infectious complications because of several femoral vein accesses she re-started PD in May 2011, but an extensive pleural effusion rapidly developed and she was referred to our unit for HD catheter placement. An angiotomography confirmed complete occlusion of jugular, subclavian, femoral and iliac veins with a patent inferior vena cava. A translumbar 40cm HD cateter (Split Stream, Medcomp, Harleysville, PA, USA) was placed percutaneously in the right atrium under general anaesthesia and radiological guidance (Fig. 3).
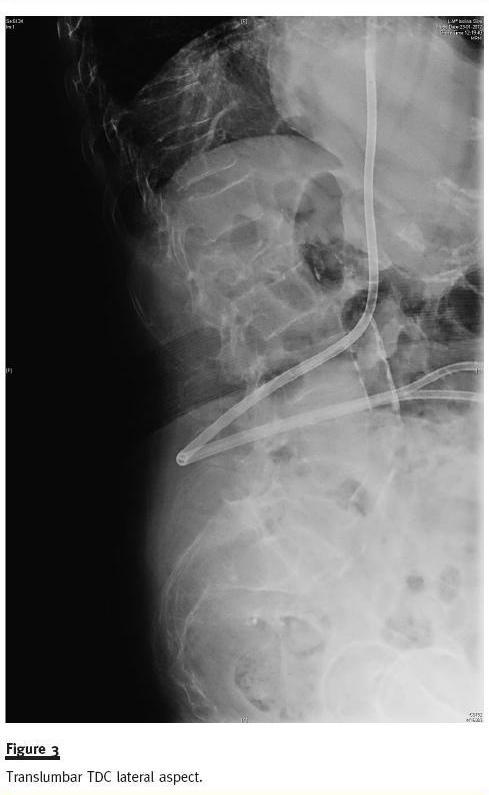
The patient was placed in a ventral decubitus position and an 18Gx20cm needle was used to perform a puncture about 10cm from the midline and 3cm above the right iliac crest. Needle direction was a 40º angle in a cephalic and medial direction. When the needle surpassed the vertebra midline, it was gently removed while performing aspiration. When blood was encountered, endovascular contrast (Visipaque 270, GE Healtcare Ireland, Cork, Ireland) was administered through the needle to confirm positioning in the inferior vena cava (contrast was seen flowing in the atrial direction). A 0.035-inch guidewire was placed and the catheter was placed though the guidewire after sequential dilation. The catheter tunnel was made laterally so the exterior exit site would be as near to the right flank as possible for patient comfort. She was placed under anticoagulation after this procedure to avoid further thrombotic complications.
The patient died due to sepsis, attributed to catheter infection, after ten months of treatment through this vascular access. No other major complications were reported except an exit site infection immediately after placement.
DISCUSSION
The cases described represent an ever-more frequent scenario in our dialysis units. In such extreme circumstances we are sometimes required to consider unusual but effective solutions to resolve our patients problems.
The EBPG recognises placement of arteriovenous fistulas as the first option for HD vascular access, followed by grafts and finally cuffed catheters4. These recommendations are based on various studies that emphasise the increased mortality, morbidity and costs associated with catheter usage for HD delivery5.
Moreover, cost analysis performed by USRDS shows higher average cost per-patient-per year to be associated with patients receiving treatment through catheters.
Nonetheless, in some cases autologous vascular accesses are not possible and a catheter is the only option. Sites available for catheter placement are finite and should be used sparingly; however, the frequent complications associated with longdwelling catheters, such as infection and thrombosis, inevitably lead to venous patrimony losses through time and can result in situations such as the ones described above6.
Long-term HD is associated with central vein stenosis (CVS), which has an incidence of 25-40%2.
Major risk factors for CVS development are previous central catheter, pacemaker or defibrillator wires and peripherally inserted central catheters. Catheter exchange through a guidewire may conserve a venous entry site indefinitely; however, infection or catheter removal due to a patent fistula or graft will lead to subsequent loss of this site. It has also been recorded that 27% of patients with CVS had a previous history of central venous catheterisation, which interferes with native access maturation and usage2.
Multiple sites are available for tunnelled dialysis catheter (TDC) placement. Their order of usage should reflect the complication rate associated with a particular site in order to prevent venous wastage. The first option should always be the right internal jugular vein, since it is associated with the best blood flow and inferior CVS incidence due to its straight course.
Left internal jugular veins have similar infection rates, but are associated with a higher placement-complication rate, dysfunction and CVS due to the tortuous path they present, which may result in damage to the vessel wall during catheterisation6. Subclavian vein catheters, although popular in the past, should always be avoided since they are prone to CVS development, occurring in up to 50% of cases7.
However, in patients presenting with bilateral jugular vein thrombosis and no permanent vascular access placement perspective, it should be the preferred site for catheter placement. Femoral vein catheters are usually considered only when all other options fail. Even using 55cm long TDC blood flow may be impaired by mechanical bending of the catheter, thrombosis and by the high probability of infection associated with this site (0.24/100 catheter days)8.
Femoral and iliac vein stenosis is also very frequent, especially after 4 weeks of permanence (29%), yet these lesions are not always clinically relevant9.
In some cases, however, the preferred sites for catheter placement are not available. In such cases, other locations as external jugular vein, external iliac vein, vena cava and suprahepatic veins may be suitable for percutaneous placement of TDC. Although the surgical approach may be considered, percutaneous placement of TDC is less invasive.
External jugular vein TDC insertion is a viable option after internal jugular vein thrombosis. There are numerous centres performing this procedure, which is safe and has a low incidence of infection (0.08/100 catheter days), comparable to that of the internal jugular vein10. A problem with this technique is associated with difficulty in transposition of the external jugular – subclavian vein junction, which may be amenable with image guidance.
External iliac vein placement of TDC is considered in some centres as a viable alternative to femoral catheters, offering the theoretical advantages of lower infection rates and better blood flow. These advantages are due to an exit site away from the groin and a catheter curve above the area hindered by leg movement, which could result in kinking and stretching of the catheter releasing heparin and promoting lumen clotting.11 In a small study with 6 patients, Betz et al.11 followed these catheters for over 1500 days without any infection recorded and malfunction resolved with antifibrinolytic therapy without any need for catheter exchange.
Translumbar catheters and suprahepatic vein catheters are last-resort catheters. Both these approaches should be made under obligatory radiological guidance.
Translumbar catheter placement is a relatively safe procedure that can be performed under local or general anaesthesia. Specific complications of this procedure are right renal artery or vein catheterisation, caudal migration of the catheter – which usually only happens in small-bore catheters and obese patients – and spontaneous retroperitoneal bleeding12.
The rates of thrombosis, fibrin sheath formation and infection are similar to other traditional sites13.
Transhepatic TDC are associated with numerous complications, some similar to others, such as bleeding and infection, and some particular to this approach, such as biliary tract communication, hepatic dysfunction and catheter migration. Contraindications for this access site are ascites and coagulopathy.
Complication rates of 29% have been reported for the placement procedure; 31% of patients had early catheter dislodgment and 19% experienced early catheter failure (<7 days)14. As such, these should only be considered in patients with no other viable options.
The cases herein presented represent situations without usual solutions for adequate dialysis delivery.
Both patients were unsuitable for urgent transplantation and had an uncommon condition that prevented effective switch to peritoneal dialysis. Planning is essential in these cases with a good characterization of the available venous tract. We subsequently chose the more distal solution available for each patient, trying to spare as much as we could of the sparse venous patrimony available since it may be life saving in the future if the need arises. We had a long follow-up without any major dysfunction, which, associated with other reports in the literature, gives us confidence to provide such options to our patients as viable ones.
The rationale behind the subcutaneous placement of the PD catheter tip was that it could be used as a last resort in a future case of catheter dysfunction or incapacity to obtain another vascular access, enabling us to initiate peritoneal dialysis. Of note, although this patient had prostate cancer, his condition was well controlled and the patient had an overall life expectancy limited by dialysis problems rather than by oncologic problems.
Although anticoagulation is frequently used to prevent TDC malfunction, randomised controlled studies supporting this practice have conflicting results and use different methods. Minidose warfarin (1mg/day) was not associated with better unassisted patency of TDC, albeit that it showed that INR<1.0 was associated with an increased risk of dysfunction and earlier need of thrombolytic therapy15. A second study compared placebo to low-intensity warfarin therapy (INR 1.5-1.9) in newly placed TDC without showing any benefit16. A study by Coli et al.17 compared warfarin (INR 1.8-2.5) and ticlopidine (250mg/day) in primary prevention to a control group with ticlopidine that would only receive warfarin for secondary prevention. The control group showed worse results with a higher rate of TDC thrombosis and dysfunction.
A pitfall of this study was that the primary prevention group achieved target INR in 92% vs. 65% in the secondary prevention group (P< 0.05).
None of the above studies had major bleeding complications.
Fibrin sleeve formation is a common complication of TDC. It becomes problematic when it covers the catheter tip preventing adequate blood flow. Clinical suspicion should arise when infusion through the ports is possible but heavy resistance is felt when blood aspiration is performed. It should also be considered when fibrinolytic administration is not capable of restoring satisfactory blood flows or results are very short lived. Treatment can be performed through fibrinolytic administration, percutaneous fibrin sheath stripping using a wire snare device or catheter exchange and sheath disruption through balloon angioplasty. These procedures, however, show mixed results, with poor long standing patency18.
Though, dialysis discontinuation may be considered in patients with exhausted vascular access patrimony, it may be problematic and difficult to accept by patients (and their families) mentally competent and with relative good quality of life. In fact, the patients presented in this paper were both mentally competent and were capable of maintaining physical autonomy, namely helping their families in domestic activities. Anyway, the possibility of suspending dialysis and/or the technical difficulty to obtain an access should always be discussed with the patients and their families. These points were exhaustively discussed with our patients, and subsequently with their families, and both accepted the risk associated to the placement of this type of catheter. Finally, the authors defend that although these types of catheters are not ideal, they should be considered in particular cases, such as patients with exhausted vascular access patrimony, or as a bridge for renal transplantation or even for PD.
With these cases our aim is to increase awareness among our nephrology community of novel access sites for central veins which could be life saving for our patients.
References
1. Allon M, Lok CE. Dialysis fistula or graft: the role for randomized clinical trials. Clin J Am Soc Nephrol 2010;5(12): 2348-2354 [ Links ]
2. Kundu S. Central venous disease in hemodialysis patients: prevalence, etiology and treatment. J Vasc Access 2010;11(1): 1-7 [ Links ]
3. Moncrief JW, Popovich RP, Broadrick LJ, He ZZ, Simmons EE, Tate RA. The Moncrief-Popovich catheter. A new peritoneal access technique for patients on peritoneal dialysis. ASAIO J 1993;39(1): 62-65 [ Links ]
4. Tordoir J, Canaud B, Haage P, et al. EBPG on Vascular Access. Nephrol Dial Transplant 2007; 22 (Suppl. 2): ii88-117 [ Links ]
5. Rayner HC, Pisoni RL, Bommer J, et al. Mortality and hospitalization in haemodialysis patients in five European countries: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 2004;19(1):108-120 [ Links ]
6. Vats HS. Complications of catheters: tunneled and nontunneled. Adv Chronic Kidney Dis 2012;19:188-194 [ Links ]
7. Cimochwski GE, Worley E, Rutherford WE, Sartain J, Blondin J, Harter H. Superiority of the internal jugular over the subclavian access for temporary dialysis. Nephron 1990;54(2):154-161 [ Links ]
8. Zaleski GX, Funaki B, Lorenz JM, et al. Experience with tunneled femoral hemodialysis catheters. AJR Am J Roentgenol 1999;172(2):493-496 [ Links ]
9. Weyde W, Badowski R, Krajewska M, Penar J, Moron K, Klinger M. Femoral and iliac vein stenoses after prolonged femoral vein catheter insertion. Nephrol Dial Transplant 2004;19(6):1618-1621 [ Links ]
10. Natário A, Barreto C, Farinha A, Parreira L, Vinhas J. Haemodialysis tunneled catheters in the external jugular vein. A single-centre experience of an alternative approach. Port J Nephrol Hypert 2010;24:279-286 Not found in PubMed [ Links ]
11. Betz C, Kraus D, Müller C, Geiger H. Iliac cuffed tunneled catheters for chronic haemodialysis vascular access. Nephrol Dial Transplant 2006;21(7):2009-2012 [ Links ]
12. Rajan DK, Croteau DL, Sturza SG, Harvill ML, Mehall CJ. Translumbar placement of inferior vena caval catheters: a solution for challenging hemodialysis access. Radiographics 1998;18(5):1155-1167 [ Links ]
13. Lund GB, Trerotola SO, Scheel PJ Jr. Percutaneous translumbar inferior vena cava cannulation for hemodialysis. Am J Kidney Dis 1995;25(5):732-737 [ Links ]
14. Smith TP, Ryan JM, Reddan DN. Transhepatic catheter access for hemodialysis. Radiology 2004;232(1):246-251 [ Links ]
15. Mokrzycki MH, Jean-Jerome K, Rush H, Zdunek MP, Rosenberg SO. A randomized trial of minidose warfarin for the prevention of late malfunction in tunneled, cuffed hemodialysis catheters. Kidney Int 2001;59(5):1935-1942 [ Links ]
16. Wilkieson TJ, Ingram AJ, Crowther MA, et al. Low-Intensity adjusted-dose warfarin for the prevention of hemodialysis catheter failure: a randomized, controlled trial. Clin J Am Soc Nephrol 2011;6(5):1018-1124 [ Links ]
17. Coli L, Donati G, Cianciolo G, et al. Anticoagulation therapy for the prevention of hemodialysis tunneled cuffed catheter (TCC) thrombosis. J Vasc Access 2006;7(3):118-122 [ Links ]
18. Kamper L, Piroth W, Haage P. Endovascular treatment of dysfunctional hemodialysis catheters. J Vasc Access 2010;11(4):263-268 [ Links ]
Dr Fernando Caeiro
Lisbon, Portugal E-mail: fccaeiro@gmail.com Conflict of interest statement. None declared. Received for publication: 04/12/2012 Accepted in revised form: 04/02/2013














