Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.27 no.1 Lisboa jan. 2013
Kidney transplantation in the north of Portugal: donor type and recipient time on dialysis
Transplante renal no norte de Portugal: tipo de dador e tempo de diálise dos receptores
Bruno A. Lima 1,2, Miguel Mendes1, Helena Alves1
1 Centro de Histocompatibilidade do Norte. Porto, Portugal.
2 Faculdade de Ciências, Universidade do Porto. Porto, Portugal.
ABSTRACT
Patients with end-stage renal disease who receive a kidney transplant are associated with a reduced risk of mortality compared with patients who remain on the waiting list. A longer wait time on dialysis is responsible for inferior health status and higher sensitisation to human leukocyte antigens at the time of transplantation. This leads to inferior long-term outcomes after transplantation.
The aim of the study is to analyse recipients time on dialysis and waiting times for deceased and living donors in the north of Portugal for first single-kidney transplant.
A total of 549 first single-kidney transplant recipients between 2008 and 2011 from Oporto transplantation units (Hospital de Santo António and Hospital São João) were included in our study.
Characteristics of first single-kidney transplant recipients from living and deceased donors at time of transplantation were compared. Frequencies were compared using the chi-squared test and median times were tested using the Wilcoxon rank-sum test or by the Kruskal-Wallis test.
Between 2008 and 2011 Hospital de Santo António (HSA) performed a significantly higher number of living donor kidney transplants (85.1%) than Hospital São João (HSJ) (14.9%). Both transplant units performed a similar number of deceased donor kidney transplants (49.5% performed at HSA and 50.5% at HSJ).
For both transplant units, median time on dialysis for living donor kidney transplant recipients was shorter (11 and 13 months) than for recipients of deceased donor kidneys (59.5 and 53 months, respectively, p < 0.001). Between 2008 and 2011, median waiting time distribution was very similar between HAS and HSJ deceased donor recipients (35 and 36 months, respectively).
As far as we know, this is the first study presenting kidney transplant recipients waiting times since implementation of the new Portuguese allocation system for deceased donor kidney transplant in August 2007.
Key-Words: Deceased donor kidneys; living donor kidneys; renal transplant; time on dialysis, waiting time.
RESUMO
O transplante de rim de doentes com insuficiência renal crónica está associado a um risco reduzido de mortalidade quando comparado com doentes que se mantêm em lista de espera. Períodos de tempo em diálise longos são responsáveis por piores estados de saúde e maior sensibilização destes doentes a antigénios leucocitários humanos no momento dum transplante renal.
O objectivo deste trabalho é o de analisar tempos de diálise e tempos de espera de receptores de primeiro transplante de rim de dador vivo e de dador cadáver no Norte de Portugal.
Foram incluídos neste estudo um total de 549 receptores de primeiro transplante renal das unidades de transplante do Porto (Hospital de Santo António e Hospital São João), entre 2008 e 2011.
Foram comparadas as características dos receptores de primeiro transplante renal de dador vivo e de dador cadáver à data do respectivo transplante. Proporções foram comparadas com recurso ao teste de Qui-Quadradro e as medianas dos tempos analisados foram testadas com o teste de Wilcoxon ou o teste de Kruskall-Wallis.
Entre os anos de 2008 e 2011, o Hospital de Santo António (HSA) realizou a maioria dos transplantes de dador vivo (85.1%) no Norte de Portugal quando comparado com o Hospital de São João (HSJ) (14.9%).
Ambas as unidades realizaram um número semelhante de transplantes de rim de dador cadáver (49.5% realizados no HSA e 50.5% no HSJ).
Nestas unidades de transplante (HSA e HSJ) as medianas dos tempos de diálise dos receptores de rim de dador vivo foram mais curtas (11 e 13 meses, respectivamente) do que as medianas dos receptores de rim de dador cadáver (59.5 e 53 meses, respectivamente, p<0.001). Entre 2008 e 2011, as distribuições das medianas dos tempos em lista de espera das duas unidades de transplante foram muito parecidas para os receptores de rim de dador cadáver (35 meses para o HSA e 36 meses para o HSJ).
Tanto quanto podemos saber, este é o primeiro estudo em Portugal que apresenta tempos de espera de doentes transplantados de rim, desde a implementação das novas normas para a selecção do par dadorreceptor em homotransplantação com rim de cadáver de Agosto de 2007 altura em que entraram em vigor.
Palavras-chave: Dador cadáver; dador vivo;
INTRODUCTION
Patients with end-stage renal disease (ESRD) who receive a kidney transplant are associated with a reduced risk of mortality compared with patients who remain on the waiting list, despite the increasing age and co-morbidities of recipients1. Kidney transplantation offers the greatest potential for increased survival, enhanced quality of life and lower healthcare costs2 particularly when compared with haemodialysis3; although the demand for kidneys worldwide far exceeds the available supply4-6.
The main challenges of kidney transplantation are achieving better outcomes of patients and grafts, improving recipients quality of life and increasing the number of available organs7-9.
Several programmes have been developed worldwide to increase the number of available organs, both from deceased10,11 and living donors12,13. Implementing multidisciplinary approaches improves staff communication, patient outcomes and the number of kidney transplants particularly from living donors7.
Longer waiting times are not only associated with higher waiting list mortality and morbidity, but may also lead to inferior outcomes after transplantation14,15.
Waiting time has been shown as the strongest modifiable risk factor for the outcome after kidney transplantation16,17. A longer waiting time on dialysis is also responsible for a higher sensitization to human leukocyte antigens (HLA) at time of transplantation, leading to inferior long-term outcomes after transplantation18,19.
Kidney transplantation from living donors has increased worldwide over the past years20, and is seen as a suitable option to achieve higher transplantation rates and shorter waiting times for all ESRD patients17.
Under the current Portuguese allocation system, deceased donor kidneys are primarily allocated via ABO-identical and a point system based on a combination of time on dialysis and HLA mismatching.
Additional points account for sensitised candidates, patients ages and prior transplants.
The aim of the study is to analyse recipients time on dialysis and waiting times for deceased and living donors in the north of Portugal for first single-kidney transplant.
PATIENTS AND METHODS
A retrospective analysis was performed using data from Portugals Northern Histocompatibility Centre, software for patients registries: Ambidata LabWay-LIMS®. In Oporto, there are two transplantation units: Hospital de Santo António and Hospital São João (HSA and HSJ, respectively). Both units receive ESRD patients from the north of Portugal for kidney transplantation.
A total of 549 first single-kidney transplant recipients between 2008 and 2011 from Oporto transplantation units were included in our study. Recipients younger than 18 and those presenting with clinically urgent need for transplantation were excluded from this study.
The current Portuguese kidney allocation system from deceased donors was implemented in August 2007 (Ordinance nº 6537/2007 from April 3rd) which is why data retrieval commences from 2008.
Characteristics of first single-kidney transplant recipients from living and deceased donors at time of transplantation were compared. Frequencies were compared using the chi-squared test and median times were tested using the Wilcoxon rank-sum test (two groups) or by the Kruskal-Wallis test (more than two groups). Transplant recipients waiting time was computed from date of hospital registration on waiting list (for kidney transplant from deceased donor) to transplantation21,22. Time on dialysis applies to the time that transplant recipients were on dialysis until transplantation (transplant date – the date of first dialysis)5.
A p value <0.05 was considered to be statistically significant. All statistical analyses were performed using SPSS for windows release 17.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Of 549 adult, first-time, kidney-only, transplant recipients between January 1 2008 and December 31 2011, a total of 74 recipients (13.5%) received a living donor kidney transplant (LDKT) and 475 (86.5%) received a deceased donor kidney transplant (DDKT).
Blood group frequencies and peak Panel-Reactive Antibody (PRA) at transplantation did not differ between patients who received DDKT and patients with LDKT (Table I).
Between 2008 and 2011 HSA performed a significantly higher number of LDKTs (85.1%) than HSJ (14.9%) while both transplant units performed a similar number of DDKT (49.5% performed in HAS and 50.5% in HSJ).
The proportion of male recipients was higher in LDKT (75.7%) than in DDKT (63.8%, p <0.05). When comparing age of DDKT recipients to LDKT recipients, those who received LDKT were more likely to be under 35 (39.2% vs. 11.6%) and less likely to be over 65 (1.4% vs 12.4%; p <0.001).
For both transplant units (HSA and HSJ), median time on dialysis in LDKT was lower (11 and 13 months respectively) than for recipients of DDKT (59.5 and 53 months, respectively, p <0.001).
Figure 1 shows the median time on dialysis (the whiskers delineate the 5th and 95th percentiles, the box represents the interquartile range – 25th through 75th percentiles and the horizontal lines within the boxes denote the median) is longer for recipients of DDKT kidneys than recipients of LDKT for both transplant units each year. We can also observe that median time on dialysis for recipients of DDKT was higher in 2008 and 2009 than in 2010 and 2011 for both transplant units. This could be a direct consequence of the implementation of the new allocation rules in August 2007. De-emphasising HLA matching (the main criteria for allocation prior to August 2007) in order to mandate greater importance to recipients time on dialysis in graft allocation.
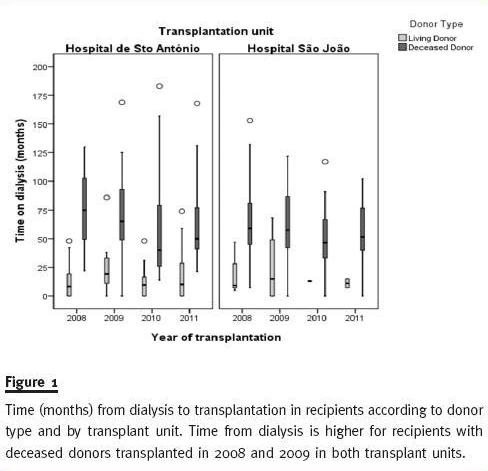
As can be seen (Table II), between 2008 and 2011, median waiting time distribution is very similar between HSA and HSJ deceased donor recipients (35 and 36 months, respectively). We believe that the difference found in 2008 between median waiting times for HSA and HSJ recipients (42 vs. 34.5, p <0.041) is just due to chance (when corrected for multiple comparisons this difference is no longer statistically significant).
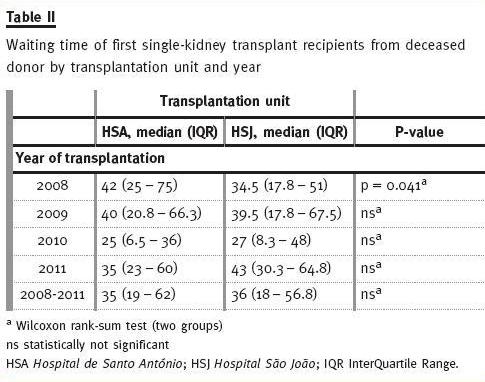
Figure 2 shows how close waiting time distribution is among recipients of DDKT within each year, between 2008 and 2011 for both HSA and HSJ.
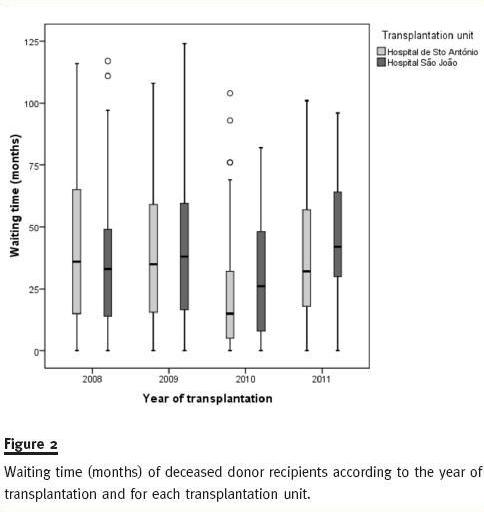
As shown in Figure 3, blood group O recipients have longer time on dialysis than patients from other blood groups. This is particularly evident in recipients with DDKT kidneys where median times on dialysis for blood group O recipients are 112, 91, 83 and 77 months in 2008, 2009, 2010 and 2011, respectively (Table III).
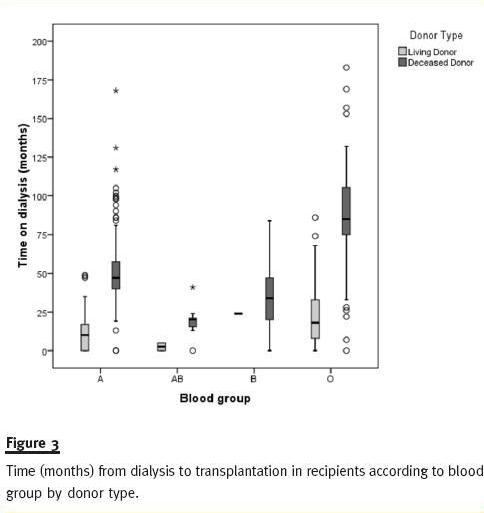
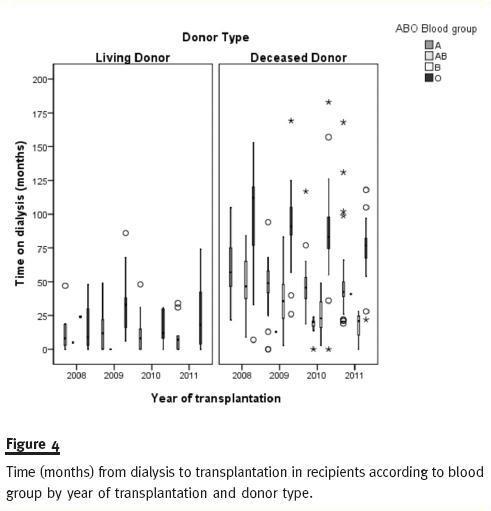
Figure 4 shows how recipients time on dialysis is higher for ABO blood type O and decreasing for all blood type DDKT recipients from 2008 to 2011.
DISCUSSION
This retrospective study shows that first-time recipients of LDKT are younger, mainly male and spend less time on dialysis than those with a deceased donor.
Before 2007, kidney allocation was performed mainly regarding HLA matching criteria and ABO compatibility. This practice resulted in longer waiting-list periods for ABO blood type O candidates.
In order to reduce wait-list time for kidney transplant candidates, 2007 rules give priority of allocation of deceased donors kidneys to ABO-identical candidates and those with longer time on dialysis.
As a result of these new kidney allocation rules, we have observed that ABO blood type O recipients of first single DDKT are those with longer time on dialysis. With the implementation of the ABO-identical rule rather than ABO compatibility, less ABO blood type O grafts are given to non-O recipients, although this is not enough to solve the blood group O problem17.
A previous study23 reported a statistically significant difference between time on dialyses of transplant recipients before and after implementation of 2007 rules. This study presented mean values for time on dialysis while we prefer to give median values for first single-kidney recipients. Medians are less sensitive to extreme scores and generally a better indicator of where the middle of the class falls. The reported increase in time on dialysis for recipients after 200723 was a goal of the new allocation system rather than an oversight. As can be seen in figure 4, recipients transplanted with deceased donors in 2008 and 2009 have longer time on dialysis than patients transplanted in 2010 and 2011, for both transplant units.
In order to reduce time on dialysis for candidates on the waiting list, it is necessary to select for transplantation those with longer time on dialysis. By doing this, candidates overall time on dialysis is shortened.
The organ donation policy for living donors in Portugal has also changed. Previously, candidates for donation could be third-degree-related donors or closer. Since June 2007 (Law No. 22/2007), anyone can be a kidney donor with the usual exceptions (minors and the mentally disturbed). Now, three quarters of the LDKT recipients are male and many of them have received grafts from their wives. Women, however, are rarely able to receive a kidney from their spouse once they have alloantibodies against their partners HLA.
Kidney transplantation is the preferred modality of treatment for chronic kidney failure. Increasing both living and deceased organ donation will allow more patients to receive kidney transplants. This not only improves the health and subsequently the quality of life of the patient but has the added benefit of being cost effective, especially when compared with haemodialysis1-3.
The greatest challenge to candidates on a deceased donor waiting list is the median time to transplantation.
The superiority of living donor grafts offers and perpetuates the best overall outcomes and furthermore living donor kidney transplant represents the most promising modality for addressing the organ crisis24.
LDKT can help to reduce time on dialysis for kidney transplant recipients. A policy of maintaining active information on living kidney donor transplantation in chronic kidney failure medical clinics and in dialysis units can significantly increase the number of transplantations25.
While LDKT represents 21.1% of all first single-kidney transplants performed in HSA, this percentage falls to 4.3% when we look at LDKT performed in HSJ. The problem of attracting a donor may lie in the unwillingness of candidates to ask potential donors or the inability of candidates to motivate said donors26. Concerns surrounding donor morbidity have been noted in patients with ESRD27.
It has been pointed that having former donors speak to potential donors could assuage these concerns, thereby improving LDKT numbers28.
Implementing an interdisciplinary supporting transplant team could increase kidney transplants, specifically those from living donors7. New approaches focussing on increasing kidney transplants from living donors should be of major concern13,29,30.
Further study in patient populations on the waiting list is needed to better inform physicians of the characteristics of those patients. A detailed characterization of kidney transplant candidates waiting list and waiting times will give us a better understanding of this issue. We need to know what the percentage of registrants receiving a transplant within a year is, or how long it takes for 50% of wait-listed candidates to receive a transplant or the rate of transplantation per year among actively listed kidney transplant candidates5,31.
In summary, the 2007 allocation law seeks fair and equal access to deceased donor kidney transplantation with the objectives of better survival rate and quality of life of patients who can benefit from a transplant. Policy makers and transplant physicians need to collect and maintain the most current information on performed kidney transplants to make effective recommendations to adjust the law.
As far as we know, this is the first study presenting kidney transplant recipients waiting times since the implementation of the new Portuguese allocation system for DDKT in August 2007.
References
1. Tonelli M, Wiebe N, Knoll G, et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant 2011;11:2093-109 [ Links ]
2. Rocha MJ, Ferreira S, Martins LS, et al. Cost analysis of renal replacement therapy by transplant in a system of bundled payment of dialysis. Clin Transplant 2012;26:529-31 [ Links ]
3. Villa G, Rodríguez-Carmona A, Fernández-Ortiz L, et al. Cost analysis of the Spanish renal replacement therapy programme. Nephrol Dial Transplant 2011;26:3709-14 [ Links ]
4. Knoll G. Trends in kidney transplantation over the past decade. Drugs 2008;68(S1):3-10 [ Links ]
5. Gill JS, Johnston O. Access to kidney transplantation: the limitations of our current understanding. J Nephrol 2007;20:501-06 [ Links ]
6. Wolfe RA, Roys EC, Merion RM. Trends in organ donation and transplantation in the United States, 1999-2008. Am J Transplant 2010;10:961-72 [ Links ]
7. Fonouni H, Golriz M, Mehrabi A, et al. The role of an interdisciplinary transplant team on living donation kidney transplantation program. Transplant Proc 2010;42:137-40 [ Links ]
8. Ketteler M, Biggar PH. Malignancies after renal transplantation. Port J Nephrol Hypert 2008;22:231-7 [ Links ]
9. Wolfe RA, McCullough KP, Schaubel DE, et al. Calculating life years from transplant (LYFT): methods for kidney and kidney-pancreas candidates. Am J Transplant 2008;8(4 Pt 2):997-1011 [ Links ]
10. Nicholson ML, Metcalfe MS, White SA, et al. A comparison of the results of renal transplantation from non-heart-beating, conventional cadaveric, and living donors. Kidney Int 2000;58:2585-91 [ Links ]
11. Audard V, Matignon M, Dahan K, Lang P, Grimbert P. Renal transplantation from extended criteria cadaveric donors: problems and perspectives overview. Transpl Int 2008;21:11-7 [ Links ]
12. Wallis CB, Samy KP, Roth AE, Rees MA. Kidney paired donation. Nephrol Dial Transplant 2011;26:2091-9 [ Links ]
13. Spital A. Increasing the pool of transplantable kidneys through unrelated living donors and living donor paired exchanges. Semin Dial 2005;18:469-73 [ Links ]
14. Noseworthy PA, Huang M, Zaltzman JS, Ramesh Prasad GV. Death with graft function in elderly patients after cadaveric renal transplantation: effect of waiting time. Transplant Proc 2004;36:2985-7 [ Links ]
15. Aalten J, Hoogeveen EK, Roodnat JI, et al. Associations between pre-kidney-transplant risk factors and post-transplant cardiovascular events and death. Transpl Int 2008;21:985-91 [ Links ]
16. Meier-Kriesche HU, Kaplan B. Waiting time on dialysis as the strongest modifiable risk factor for renal transplant outcomes: a paired donor kidney analysis. Transplantation 2002;74:1377-81 [ Links ]
17. Glander P, Budde K, Schmidt D, et al. The blood group O problem in kidney transplantation–time to change? Nephrol Dial Transplant 2010;25:1998-2004 [ Links ]
18. Wu HH, Tien YC, Huang CI, Chiang YJ, Chu SH, Lai PC. HLA class I antibodies in patients awaiting kidney transplantation and the association with renal graft survival. Transplant Proc 2008;40:2191-4 [ Links ]
19. Figueiredo A, Moreira P, Parada B, et al. Risk factors for delayed renal graft function and their impact on renal transplantation outcome. Transplant Proc 2007;39:2473-5 [ Links ]
20. Mandelbrot DA, Pavlakis M. Living Donor Practices in the United States. Adv Chronic Kidney Dis 2012;19:212-9 [ Links ]
21. Schaubel DE, Dykstra DM, Murray S, et al. Analytical approaches for transplant research, 2004. Am J Transplant 2005;5(4 Pt 2):950-7 [ Links ]
22. Wolfe RA, Schaubel DE, Webb RL, et al. Analytical approaches for transplant research. Am J Transplant 2004;4 (Suppl 9):106-13 23. [ Links ] Gonçalves JA, Jorge C, Atalaia A, et al. New law of renal transplantation in Portugal associated with more acute rejection episodes and higher costs. Transplant Proc 2012;44:2276-9 [ Links ]
24. Cecka M. Clinical outcome of renal transplantation. Factors influencing patient and graft survival. Surg Clin North Am 1998;78:133-48 [ Links ]
25. González-Monte E, Delgado I, Polanco N, et al. Results of a living donor kidney promotion program. Transplant Proc 2010;42:2837-8 [ Links ]
26. Reese PP, Shea JA, Bloom RD, et al. Predictors of having a potential live donor: a prospective cohort study of kidney transplant candidates. Am J Transplant 2009;9:2792-9 [ Links ]
27. Barnieh L, McLaughlin K, Manns BJ, Klarenbach S, Yilmaz S, Hemmelgarn BR. Barriers to living kidney donation identified by eligible candidates with end-stage renal disease. Nephrol Dial Transplant 2011;26:732-8 [ Links ]
28. Stothers L, Gourlay WA, Liu L. Attitudes and predictive factors for live kidney donation: A comparison of live kidney donors versus nondonors. Kidney Int 2005;67:1105-11 [ Links ]
29. Lima B, Dias L, Henriques A, Alves H. The Portuguese match algorithm in the kidney paired donation program. Organs, Tissues & Cells 2010;13:25-32 [ Links ]
30. Lima B, Alves H. A Portuguese living donor exchange programme. Organs, Tissues & Cells 2012;15:123-9 [ Links ]
31. Levine GN, McCullough KP, Rodgers AM, Dickinson DM, Ashby VB, Schaubel DE. Analytical Methods and Database Design: Implications for Transplant Researchers, 2005. Am J Transplant 2006;6:1228-42 [ Links ]
Dr Bruno A. Lima
Centro de Histocompatibilidade do Norte
Rua Dr. Roberto Frias
4200-465 Oporto, Portugal
E-mail: bruno@chnorte.min-saude.pt
Conflict of interest statement. None declared.
Received for publication: 02/07/2012
Accepted in revised form: 22/11/2012














