Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.27 no.1 Lisboa jan. 2013
Acute kidney injury: definition and epidemiology
Lesão Renal Aguda: definição e epidemiologia
José António Lopes
Department of Nephrology and Renal Transplantation, Hospital de Santa Maria,
Centro Hospitalar de Lisboa Norte, Lisboa, Portugal
ABSTRACT
Over the last decades, more than 35 different definitions have been used to describe acute kidney injury (AKI). Multiple definitions for AKI have obviously led to a great disparity in the reported incidence and mortality of AKI making it difficult or even impossible to compare the various published studies focusing on AKI. Therefore, it became crucial to establish a consensual and accurate definition of AKI that could desirably be used worldwide. Recent consensus criteria for AKI definition and classification [the Risk Injury Failure Loss of kidney function End-stage kidney disease (RIFLE) and the Acute Kidney Injury Network (AKIN) classifications] have led to more consistent estimates of its epidemiology. This review will present and critically discuss current literature about AKI diagnosis and epidemiology.
Key-words: Acute kidney injury; definition; epidemiology
RESUMO
Nas últimas décadas têm sido utilizadas mais de 35 definições diferentes para o diagnóstico de lesão renal aguda (LRA). As múltiplas definições levaram, obviamente, a uma disparidade importante na incidência e mortalidade observada nas publicações, tornando difícil ou mesmo impossível comparar os vários estudos sobre lesão renal aguda. Por este motivo, tornou-se crucial estabelecer uma definição consensual e precisa da LRA, que pudesse ser utilizada de forma generalizada. Critérios recentes na definição e classificação de LRA [the Risk Injury Failure Loss of kidney function End-stage kidney disease (RIFLE) and the Acute Kidney Injury Network (AKIN)] permitiram uma estimativa mais consistente da epidemiologia. Esta revisão tem por objetivo apresentar e discutir de forma crítica, a literatura atual sobre epidemiologia e diagnóstico da LRA.
Palavras-chave: Definição; epidemiologia; lesão renal aguda.
DEFINITION AND CLASSIFICATION OF AKI
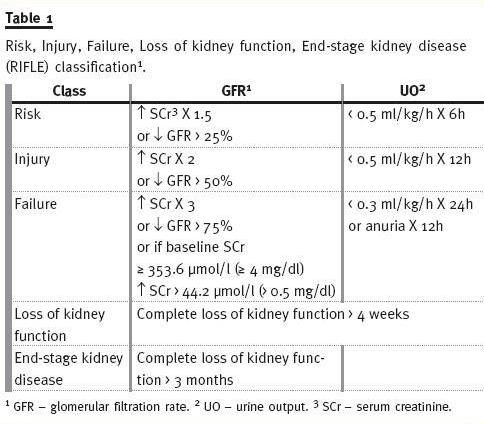
The Risk Injury Failure Loss of kidney function End-stage kidney disease (RIFLE) classification
In May 2002, the Acute Dialysis Quality Initiative (ADQI) group for the study of AKI, composed by nephrologists and intensivists, gathered during two days in a conference in Vicenza (Italy), with the purpose of defining AKI. From this conference, the consensual Risk Injury Failure Loss of kidney function End-stage kidney disease (RIFLE) classification for AKI definition emerged, and was later published in May 2004 on Critical Care1. The RIFLE classification (Table 1) is based on SCr and urine output (UO) determinations, and considers three severity classes of AKI (Risk, Injury, and Failure), according to the variations in SCr and/or UO, and two outcome classes (Loss of kidney function and End-stage kidney disease). The patient should be classified using the criteria (SCr and/or UO), which leads to the worst classification (maximum RIFLE), for instance, if a patient would be in Risk class according to UO but in Injury class according to SCr variation, then the worst criteria (SCr) should be used for classifying AKI in this patient. The temporal pattern of the SCr and/or UO variation is also relevant for defining AKI: the deterioration of renal function must be sudden (1 to 7 days) and sustained (persisting more than 24 hours). If this definition can easily be applied when baseline SCr is known, however, in a significant number of patients baseline SCr is unknown; in these cases, if there is no history of chronic kidney disease (CKD), baseline SCr should be calculated using the Modification of Diet in Renal Disease (MDRD)2 equation, assuming a baseline GFR of 75 ml/min/1.73m2. The RIFLE has been largely validated in terms of determining the incidence of AKI and its prognostic stratification in several settings of hospitalized patients3-8. In these studies, RIFLE allowed the identification of a large proportion of patients as having AKI and there was an independent and stepwise increase in mortality as AKI severity increased; RIFLE also exhibited a good prognostic accuracy in terms of mortality. Furthermore, it has been shown that RIFLE allows monitoring the progression of AKI severity within hospitalization and RIFLE classes were associated with increased length of stay, renal replacement therapy requirement, renal function recovery and discharge from hospital to a care facility4-7.
The Acute Kidney Injury Network (AKIN) classification
In September 2005, in a meeting in Amsterdam a new classification of AKI has been proposed by the Acute Kidney Injury Network (AKIN) working group composed by nephrologists, critical care physicians and other physicians specialized in AKI. The AKIN classification (Table 2) has been published in March 2007 on Critical Care9, and it is a version of the RIFLE classification with some modifications: the diagnosis of AKI is only considered after achieving an adequate status of hydration and after excluding urinary obstruction; the AKIN classification only relies on SCr and not on GFR changes; baseline SCr is not necessary in AKIN classification, and it requires at least two values of SCr obtained within a period of 48 hours; AKI is defined by the sudden decrease (in 48 hours) of renal function, defined by an increase in absolute SCr of at least 26.5 mmol/l (0.3 mg/dl) or by a percentage increase in SCr equal to or higher than 50% (1.5x baseline value), or by a decrease in UO (documented oliguria lower than 0.5 ml/kg/hour for more than 6 hours); stage 1 corresponds to Risk class, but it also considers an absolute increase in SCr equal to or higher than 26.5 mmol/l (0.3 mg/dl); stages 2 and 3 correspond to Injury and Failure classes, respectively; stage 3 also considers patients requiring RRT, independently of the stage (defined by SCr and/or UO) they are in at the moment they initiate RRT; the two outcome classes (Loss of kidney function and End-stage kidney disease) were removed from the classification.
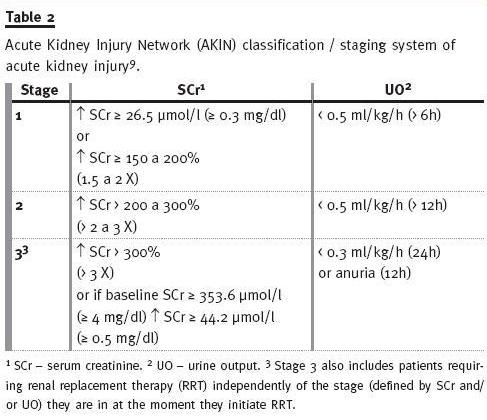
These modifications were based in the cumulative evidence that even small increases in SCr are associated with a poor outcome, and in the extreme variability of resources and of the indications to start RRT exhibited in different countries and hospitals10,11.
It has been shown that both the AKIN and the RIFLE classifications allowed the identification and stratification of AKI in a large proportion of hospitalized patients and it was independently associated with outcome12-16. In fact, patients with AKI had higher in-hospital mortality and longer lengths of stay, and AKI survivors were more likely to be discharged to an extended care facility.
The AKIN classification could theoretically improve the RIFLE criteria sensitivity and specificity, although the advantages of the RIFLE modifications have not been proven yet. In actual fact, AKIN classification compared to RIFLE classification did not exhibit a better prognostic acuity, in terms of in-hospital mortality, although it could allow the identification of more patients as having AKI13-16.
The Kidney Disease Improving Global Outcomes (KDIGO) classification
The Kidney Disease Improving Global Outcomes (KDIGO) work group has recently made the fusion of the RIFLE and AKIN classifications in order to establish one classification of AKI for practice, research, and public health. Therefore, AKI has been defined as an increase in SCr ≥ 0.3 mg/dl (≥ 26.5 mmol/l) within 48 hours; or an increase in SCr to ≥ 1.5 times baseline, which is known or presumed to have occurred within the prior 7 days; or an urine volume < 0.5 ml/kg/h for 6 hours. Furthermore, AKI has been staged in severity according to the AKIN criteria. One additional change in the criteria was made for the sake of clarity and simplicity. For patients reaching stage 3 by SCr > 4.0 mg/dl (> 354 μmol/l), rather than require an acute increase of ≥ 0.5 mg/dl (≥ 44 μmol/l) over an unspecified time period, it instead requires that the patient first achieve the creatinine based change specified in the definition [either ≥ 0.3mg/dl (≥ 26.5 μ mol/l) within a 48-hour time window or an increase of ≥ 1.5 times baseline]. This change brings the definition and staging criteria to greater parity and simplifies the criteria17.
One of the major caveats of these clinical classifications is that they rely on SCr and/or UO changes, which are insensitive and non-specific markers of kidney function. First, the endogenous production and serum release of Cr is variable, and it is influenced by multiple factors, namely age, gender, diet, and muscle mass. Second, 10 to 40% of Cr elimination is performed by tubular secretion18 and this mechanism is amplified as GFR diminishes, thus, overestimating renal function in AKI patients.
Third, many medications inhibit tubular secretion of Cr (i.e., thrimetroprim, cimetidine), causing a temporary increase in SCr. Fourth, various factors can interfere with SCr determination (i.e., acetoacetate accumulated in diabetic ketoacidosis can interfere with the alcaline picrate method), originating a false elevation in SCr19. Fifth, Cr is a marker of renal function, and not of renal lesion. Sixth, sensitivity and specificity of UO can be significantly changed by diuretics use. Seventh, UO can only be determined in patients with a bladder catheter in place, which, despite being common in ICU patients, is not frequent in other hospitalized patients. Furthermore, in CKD patients, compared with patients with previously normal renal function, the percentual increase in SCr used to define AKI generally occurs later and, thus, defining AKI by employing exclusively SCr criteria could diminish sensitivity of AKI diagnosis in CKD patients20.
New biomarkers of AKI
Remarkably, clinical classifications of AKI also do not provide any information regarding the origin of the renal lesion (i.e., cellular or subcelular levels), as opposed to several biomarkers of AKI recently identified and studied. Furthermore, the limitations of the conventional renal function markers (Cr and UO) can be overwhelmed with the utilization of those new biomarkers. In fact, various urinary and serum markers of AKI have been identified by new techniques based on proteomics21, such as cystatin C, neutrophil gelatinase-associated lipocalin (NGAL), interleukin-18 (IL-18) and the kidney injury molecule-1 (KIM-1). Current state of the art does not allow declaring which of these biomarkers is the ideal one: however, a biochemical pattern can be drawn basing on the different features of each of them. In many pivotal studies biomarkers showed to elevate soon in AKI (1 to 3 days before the increase in SCr)22. Others appeared to reflect different aetiologies of renal injury23.
Then, they seemed to dynamically change with treatment or recovery, which suggests that they can be used to monitor interventions24. In recent studies, subpopulations of patients who did not have AKI, according to SCr-based criteria, but actually had a degree of subclinical renal damage, were identified by biomarkers and associated with worse outcomes25. Finally, by identifying possible mechanisms of injury, these biomarkers might increase our understanding of the pathogenesis of AKI and/or help in prognosis/triaging of AKI syndrome26.
Although urinary NGAL and cystatin C are probably the most studied renal biomarkers, multiple other molecules are currently under investigation. No study so far, however, attempted to show a positive association of biomarkers use (and relative cost) with hard clinical outcomes. It must finally be remembered that apart from biomarkers expectations, the operators never have to forget AKI causes and pathophysiology: the current effort to standardize AKI and to diagnose it with magic tools before it can be clinically manifest will never be able to track AKI before renal damage has even occurred.
Furthermore, any diagnostic approach to the cause or trigger of AKI must take into account the local context and epidemiology. In this light, each critically ill patient should be closely observed, AKI occurrence never overlooked and pathophysiology/prevention.
EPIDEMIOLOGY OF AKI
Incidence and mortality
Large epidemiological studies have suggested that the occurrence of AKI is rising over time27-29. Older age, much higher frequency of comorbidities, diagnostic and therapeutic interventions, increased prevalence of human immunodeficiency virus infection and related treatments, more frequent non-renal organ transplantations, and more aggressive diagnostic and therapeutic interventions can contribute to this phenomenon30. Another explanation is the increasing prevalence of diabetes patients who are increasingly being treated with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and aldosterone antagonists, which all carry an increased risk for AKI, particularly during episodes of dehydration31,32. In addition, the increased awareness on the part of non-nephrologist ICU specialists of the potential presence of AKI has probably also increased. This increased awareness has been accompanied by a more widespread use of so-called non-renal indications for dialysis and an earlier start of dialysis in ICU patients33,34.
Additionally, the aforementioned studies have also revealed a declining trend in mortality27-29. Whether this decline can be attributed to an improvement in the overall care of patients or to specific interventions or therapies aimed at those with AKI remains unknown35,36. This decline in mortality has, however, occurred despite reported changes to the clinical profile and characteristics of patients with AKI37,38.
Observational studies suggest that patients with AKI are increasingly older, have more comorbid diseases, are more probably septic, and have greater severity of illness and organ failure4,5,16. Nevertheless, there has been considerable controversy as to whether the clinical outcomes – in particular, mortality associated with AKI – have improved. For example, in a systematic review mortality associated with AKI has shown no consistent change over several decades39. Regrettably, their study was highly prone to bias and was limited by only reporting crude mortality rates across those studies included and by the inability to show equivalent illness severity. Recent larger epidemiological studies examining the incidence of AKI (defined by the recently proposed consensus criteria) and its impact on mortality have found that AKI is an alarmingly common disorder in hospitalized patients, particularly in the critically ill, leading to decreased survival. Furthermore, these studies pointed towards a graded relationship of increased mortality with increasing severity of AKI.
Uchino and colleagues focused on the predictive ability of the RIFLE classification in a cohort of 20,126 patients admitted to a teaching hospital for > 24 h over a 3-year period3. Acute kidney injury occurred in 18% of patients, as follows: 9.1% were on class Risk, 5.2% on class Injury and 3.7% on class Failure.
There was a nearly linear increase in hospital mortality with increasing RIFLE class, with patients at Risk having more than three times the mortality rate of patients without AKI. Patients with Injury had close to twice the mortality of Risk, and patients with Failure had 10 times the mortality rate of hospitalized patients without AKI. On multivariate analysis, RIFLE classification was an independent predictor of mortality: class Risk carried an odds ratio of hospital mortality of 2.5, Injury of 5.4 and Failure of 10.1. In a larger single-centre multi-ICU study, the occurrence of AKI was examined in 5383 critically ill patients4.
AKI occurred in 67 % of patients, with 12 % achieving a maximum class of Risk, 27 % Injury and 28 % Failure. Of the 1,510 patients who reached Risk, 56 % progressed to either Injury or Failure. Patients with a maximum score of Risk had a mortality rate of 8.8 %, compared to 11.4 % for Injury and 26.3 % for Failure. On the other hand, patients who had no evidence of AKI had a mortality rate of 5.5 %. Furthermore, after adjusting for other covariates with prognostic impact class Injury (adjusted hazard ratio of 1.4) and class Failure (adjusted hazard ratio of 2.7) were independent predictors of hospital mortality.
In two recent multicentre studies including more than 160,000 patients very similar results were found. First, Ostermann and Chang analysed 41,972 patients admitted to 22 intensive care units (ICUs) in the United Kingdom and Germany, between 1989 and 1999, as part of the Riyadh Intensive Care Program database5. AKI defined by RIFLE occurred in 15,019 (35.8%) patients: 7,207 (17.2 %) with Risk, 4,613 (11%) Injury and 3,199 (7.6%) with Failure. Hospital mortality rates were higher in patients with AKI than in patients with no AKI, and increased in accordance with severity of AKI (no AKI 8.4%, Risk 20.9%, Injury 45.6% and Failure 56.8%). Class Risk (adjusted odds ratio 1.40), class Injury (adjusted odds ratio 1.96) and class Failure (adjusted odds ratio 1.59); were independent risk factors of in-hospital mortality.
Interestingly, renal replacement therapy for AKI was not an independent risk factor for hospital mortality.
More recently, Bagshaw and colleagues have reported on data from the Australian New Zealand Intensive Care Society Adult Patient Database7. They evaluated 120,123 patients admitted, from the 1st January 2000 to the 31st December 2005, to 57 ICUs across Australia.
The RIFLE criteria for AKI on the day of admission occurred in 36.1% of patients with a maximum RIFLE category of Risk in 16.3%, Injury in 13.6% and Failure in 6.3%. Acute kidney injury was associated with an increase in hospital mortality (adjusted odds ratio 3.29) and hospital mortality stratified by RIFLE category was 17.9% for Risk, 27.7% for Injury and 33.2% for Failure. By multivariate analysis, each RIFLE category was independently associated with hospital mortality (adjusted odds ratio: Risk 1.58, Injury 2.54 and Failure 3.22). In a retrospective observational study, Thakar and colleagues examined the effect of severity of AKI (defined by the AKIN criteria) or renal recovery on risk-adjusted mortality among 325,395 critically ill patients admitted to 191 US Veterans Affairs ICUs across the country40. Overall, 22% (N = 71,486) of patients developed acute kidney injury (Stage I: 17.5%; Stage II: 2.4%; Stage III: 2%); 16.3% patients met AKI criteria within 48 hrs, with an additional 5.7% after 48 hrs of ICU admission. Acute kidney injury frequency varied between 9% and 30% across ICU admission diagnoses.
After adjusting for severity of illness in a model that included urea and creatinine on admission, odds of death increased with increasing severity of AKI: Stage I, adjusted odds ratio 2.2; Stage II, adjusted odds ratio 6.1; and Stage III, adjusted odds ratio 8.6. Acute kidney injury patients with sustained elevation of creatinine experienced higher mortality risk than those who recovered. The discrepancies observed in the incidence and mortality in the aforementioned studies could reflect differences in case-mix, and experience with treatment of AKI and its concomitant pathology.
The negative impact of AKI on patient outcome still persists after hospitalization. In fact, patients who survive AKI have a greater rate of long-term mortality and other adverse outcomes (i.e., progression to or acceleration of CKD and cardiovascular disease) than patients who survive hospitalization without AKI in varied settings, such as community/hospitalized patients, hospitalized patients and ICU patients41,42. Therefore, understanding the impact of AKI on long-term outcomes will have a marked impact on treatment and risk stratification during hospitalization and will assist with guiding follow-up care after discharge.
Extra-renal effects of AKI, impaired immunity and progression to or acceleration of CKD
There is increasing knowledge of AKI and deleterious interorgan crosstalk that arises, at least in part, due to the imbalance of immune, inflammatory, and soluble mediator metabolism that attends severe insults to the kidney (i.e., heart, lungs, brain, liver, and other organs)43,44,45,46. In addition, there is also evidence that AKI impairs innate immunity and is associated with higher infection rates5-7,47. Such effects are likely to contribute to the poor survival of patients suffering from AKI despite excellent electrolyte, acid-base, volume, and small solute clearance. Critical reappraisal of current literature on experimental studies, generally conducted on models of ischaemia reperfusion, revealed multiple pathways and mechanisms of organ injury: local activation of the coagulation system48 infiltration of the kidney by leukocytes49, endothelial injury50, expression of adhesion molecules51, release of cytokines52, induction of toll-like receptors53, activation of intra-renal vasoconstrictor pathways54 and induction of apoptosis55.
There are also associated changes in tubular cells with loss or inversion of polarity56 and loss of adhesion to the basement membrane57.
After an episode of AKI, it is likely that there is failure to resolve renal structure and function adequately58,59.
Acute kidney injury itself may increase the risk of subsequent events and decrease kidney reserve leading to an increased risk of CKD or progression of CKD in patients with previous renal impairment41,60,61. Through inflammatory and fibrotic signaling pathways, residual kidney damage can lead to progressive structural kidney damage, which may then predispose to worsening hypertension, proteinuria and more rapid decreases in GFR62-64, all well-known risk factors for cardiovascular disease65-67. The progression for CKD may account for the burden of cardiovascular disease and mortality of patients who had AKI during hospitalization.
Recently, it was found an association between kidney function decline after a non-dialysis requiring AKI and long-term mortality in ICU survivors68.
CONCLUSIONS
The mortality of AKI remains unacceptably high. It is possible that even a short and mild episode of AKI may contribute to patient morbidity and mortality. Thus, this complex syndrome should be prevented, aggressively treated and never overlooked, even the milder forms. AKI survivors have to be closely monitored after discharge since AKI is associated with progression to or acceleration of CKD, extra-renal organ dysfunction and, therefore, with long-term morbidity and mortality. Taking into consideration that immune and inflammatory pathways likely contribute to poor survival of patients with AKI novel immune therapeutics can improve outcomes from AKI.
REFERENCES
1. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P and the ADQI workgroup. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8:R204 [ Links ]
2. Manjunath G, Sarnak MJ, Levey AS. Prediction equations to estimate glomerular filtration rate: an update. Curr Opin Nephrol Hypertens 2001; 10:785-792 [ Links ]
3. Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med 2006; 34:1913-1917 [ Links ]
4. Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care 2006; 10:R73 [ Links ]
5. Ostermann M, Chang RW. Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med 2007; 35:1837-1843 [ Links ]
6. Cruz DN, Bolgan I, Perazella MA, et al. North East Italian Prospective Hospital Renal Outcome Survey on Acute Kidney Injury (NEiPHROS-AKI): targeting the problem with the RIFLE criteria. Clin J Am Soc Nephrol 2007; 2:418-425 [ Links ]
7. Bagshaw SM, George C, Dinu I, Bellomo R. A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol Dial Transplant 2008; 23:1203-1210 [ Links ]
8. Lopes JA, Jorge S. The RIFLE and AKIN classifications for acute kidney injury: a critical and comprehensive review. Clin Kidney J 2013; 6:8-14 [ Links ]
9. Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11:R31 [ Links ]
10. Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. JAMA 1996; 275:1489-1494 [ Links ]
11. Lassnigg A, Schmidlin D, Mouhieddine M, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: A prospective cohort study. J Am Soc Nephrol 2004; 15: 1597-1605 [ Links ]
12. Barrantes F, Feng Y, Ivanov O, et al. Acute kidney injury predicts outcomes of noncritically ill patients. Mayo Clin Proc 2009; 84:410-416 [ Links ]
13. Bagshaw SM, George C, Bellomo R, for the ANZICS Database Management Committee. A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transplant 2008; 23:1569-1574 [ Links ]
14. 15. Haase M, Bellomo R, Matalanis G, Calzavacca P, Dragun D, Haase-Fielitz A. A comparison of the RIFLE and Acute Kidney Injury Network classifications for cardiac surgeryassociated acute kidney injury: a prospective cohort study. J Thorac Cardiovasc Surg 2009; 138:1370-1376 [ Links ] 16. Lopes JA, Fernandes P, Jorge S, et al. Acute kidney injury in intensive care unit patients: a comparison between the RIFLE and the Acute Kidney Injury Network classifications. Crit Care 2008; 12:R110 [ Links ] 17. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int 2012; 2:S1-138 [ Links ] 18. Shemesh O, Golbetz H, Kriss JP, Myers BD. Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int 1985; 28:830-838 [ Links ] 19. Molitch ME, Rodman E, Hirsch CA, Dubinsky E. Spurious serum creatinine elevations in ketoacidosis. Ann Intern Med 1980; 93:280-281 [ Links ] 20. Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol 2009; 20:672-679 [ Links ] 21. Venkataraman R, Kellum JA. Defining acute renal failure: the RIFLE criteria. J Intensive Care Med 2007; 22:187-193 [ Links ] 22. Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2005; 365:1231-1238 [ Links ] 23. Ricci Z, Cruz DN, Ronco C. Classification and staging of acute kidney injury: beyond the RIFLE and AKIN criteria. Nat Rev Nephrol 2011; 7:201-208 [ Links ] 24. Ricci Z, Luciano R, Favia I, et al. High-dose fenoldopam reduces postoperative neutrophil gelatinase-associated lipocaline and cystatin C levels in pediatric cardiac surgery. Crit Care 2011: 15:R160 [ Links ] 25. Haase M, Devarajan P, Haase-Fielitz A, et al. The outcome of neutrophil gelatinaseassociated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol 2011; 57:1752-1761 [ Links ] 26. Kümpers P, Hafer C, Lukasz A, et al. Serum neutrophil gelatinase-associated lipocalin at inception of renal replacement therapy predicts survival in critically ill patients with acute kidney injury. Crit Care 2010; 14:R9 [ Links ] 27. Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM. Declining mortality in patients with acute renal failure, 1998 to 2002. J Am Soc Nephrol 2006; 17:1143-1150 [ Links ] 28. Xue JL, Daniels F, Star RA, et al. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol 2006; 17:1135-1142 [ Links ] 29. Bagshaw SM, George C, Bellomo R for the ANZICS Database Management Committee. Changes in the incidence and outcome for early acute kidney injury in a cohort of Australian intensive care units. Crit Care 2007; 11:R68 [ Links ] 30. Lameire N, Van Biesen W, Vanholder R. The changing epidemiology of acute renal failure. Nat Clin Nephrol 2006; 2:364-377 [ Links ] 31. McGuigan J, Robertson S, Isles C. Life threatening hyperkalaemia with diarrhoea during ACE inhibition. Emerg Med J 2005; 22:154-155 [ Links ] 32. Stirling C, Houston J, Robertson S, et al. Diarrhoea, vomiting and ACE inhibitors: An important cause of acute renal failure. J Hum Hypertens 2003; 17: 419-423 [ Links ] 33. Kellum JA, Bellomo R, Mehta R, Ronco C. Blood purification in non-renal critical illness. Blood Purif 2003; 21: 6-13 [ Links ] 34. Ricci Z, Ronco C, DAmico G, et al. Practice patterns in the management of acute renal failure in the critically ill patient: An international survey. Nephrol Dial Transplant 2006; 21: 690-696 [ Links ] 35. Jörres A, Gahl GM, Dobis C, et al. Haemodialysis-membrane biocompatibility and mortality of patients with dialysis-dependent acute renal failure: a prospective randomized multicentre trial. International Multicentre Study Group. Lancet 1999, 354:1337-1341 [ Links ] 36. Ronco C, Bellomo R, Homel P, et al. Effects of different doses in continuous venovenous haemofiltration on outcomes of acute renal failure: a prospective randomized trial. Lancet 2000: 356: 26-30 [ Links ] 37. Liaño F, Junco E, Pascual J, Madero R, Verde E. The spectrum of acute renal failure in the intensive care unit compared with that seen in other settings. The Madrid Acute Renal Failure Study Group. Kidney Int Suppl 1998: 66: S16-S24 [ Links ] 38. Bellomo R. The epidemiology of acute renal failure: 1975 versus 2005. Curr Opin Crit Care 2006: 12: 557-560 [ Links ] 39. Ympa YP, Sakr Y, Reinhart K, Vincent JL. Has mortality from acute renal failure decreased? A systematic review of the literature. Am J Med 2005; 118: 827-832 [ Links ] 40. Thakar CV, Christianson A, Freyberg R, Almenoff P, Render ML. Incidence and outcomes of acute kidney injury in intensive care units: a Veterans Administration study. Crit Care Med 2009; 37: 2552-2558 [ Links ] 41. Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and metaanalysis. Am J Kidney Dis 2009;53: 961-973 [ Links ] 42. Morgera S, Schneider M, Neumayer HH. Long-term outcomes after acute kidney injury. Crit Care Med 2008;36(Suppl): S193-S197 [ Links ] 43. Kelly KJ. Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol 2003; 14:1549-1558 [ Links ] 44. Paladino JD, Hotchkiss JR, Rabb H. Acute kidney injury and lung dysfunction: a paradigm for remote organ effects of kidney disease? Microvasc Res 2009; 77:8-12 [ Links ] 45. Liu M, Liang Y, Chigurupati S, et al. Acute kidney injury leads to brain inflammation and functional changes in the brain. J Am Soc Nephrol 2008; 19:1360-1370 [ Links ] 46. Li X, Hassoun HT, Santora R, Rabb H. Organ crosstalk: the role of the kidney. Curr Opin Crit Care 2009; 15:481-487 [ Links ] 47. Jang HR, Rabb H. The innate immune response in ischemic acute kidney injury. Clin Immunol 2009; 130:41-50 [ Links ] 48. Thuillier R, Favreau F, Celhay O, Macchi L, Milin S, Hauet T. Thrombin inhibition during kidney ischemia-reperfusion reduces chronic graft inflammation and tubular atrophy. Transplantation 2010; 90:612-621 [ Links ] 49. Versteilen AM, Blaauw N, Di Maggio F, et al. Rho-kinase inhibition reduces early microvascular leukocyte accumulation in the rat kidney following ischemia-reperfusion injury: roles of nitric oxide and blood flow. Nephron Exp Nephrol 2011; 118:e79-e86 [ Links ] 50. Kwon O, Hong SM, Ramesh G. Diminished NO generation by injured endothelium and loss of macula densa nNOS may contribute to sustained acute kidney injury after ischemia-reperfusion. Am J Physiol Renal Physiol 2009; 296:F25-F33 [ Links ] 51. Kato N, Yuzawa Y, Kosugi T, et al. The E-selectin ligand basigin/CD147 is responsible for neutrophil recruitment in renal ischemia/reperfusion. J Am Soc Nephrol 2009; 20:1565-1576 [ Links ] 52. Thurman JM. Triggers of inflammation after renal ischemia/reperfusion. Clin Immunol 2007; 123:7-13 [ Links ] 53. Pulskens WP, Teske GJ, Butter LM, et al. Toll-like receptor-4 coordinates the innate immune response of the kidney to renal ischemia/reperfusion injury. PLoS One 2008; 3:e3596 [ Links ] 54. Conger DJ. Vascular alterations in acute renal failure: roles of initiation and maintenance. In: Molitoris BA, Finn WF, eds. Acute renal failure: a companion to Brenner and Rectors the kidney, 1st edn. Philadelphia: WB Saunders 2001; 13-29 [ Links ] 55. Saikumar P, Venkatachalam MA. Role of apoptosis in hypoxic/ischemic damage in the kidney. Semin Nephrol 2003; 23:511-521 [ Links ] 56. Zuk A, Bonventre JV, Brown D, Matilin KS. Polarity, integrin, and extracellular matrix dynamics in the post-ischemic rat kidney. Am J Physiol 1998; 275:C711-C731 [ Links ] 57. Abuelo JG. Normotensive ischemic acute renal failure. N Engl J Med 2007; 357:797-805 [ Links ] 58. Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 2001; 281:F887-F899 [ Links ] 59. Basile DP. Rarefaction of peritubular capillaries following ischemic acute renal failure: a potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens 2004; 13:1-7 [ Links ] 60. Block CA, Schoolwerth AC. The epidemiology and outcome of acute renal failure and the impact on chronic kidney disease. Semin Dial 2006; 19:450-454 [ Links ] 61. Basile C. The long-term prognosis of acute kidney injury: acute renal failure as a cause of chronic kidney disease. J Nephrol 2008; 21:657-662 [ Links ] 62. Spurgeon-Pechman KR, Donohoe DL, Mattson DL, Lund H, James L, Basile DP. Recovery from acute renal failure predisposes hypertension and secondary renal disease in response to elevated sodium. Am J Physiol Renal Physiol 2007; 293:F269-F278 [ Links ] 63. Basile DP. The endothelial cell in ischemic acute kidney injury: Implications for acute and chronic function. Kidney Int 2007; 72:151-156 [ Links ] 64. Eddy AA. Progression in chronic kidney disease. Adv Chronic Kidney Dis 2005; 12:353-365 [ Links ] 65. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351:1296-1305 [ Links ] 66. Sarafidis PA, Bakris GL. Microalbuminuria and chronic kidney disease as risk factors for cardiovascular disease. Nephrol Dial Transplant 2006; 21: 2366-2374 [ Links ] 67. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903-1913 [ Links ] 68. Lai CF, Wu VC, Huang TM, et al. Kidney function decline after a non-dialysis-requiring acute kidney injury is associated with higher long-term mortality in critically ill survivors. Crit Care 2012; 16:R123 [ Links ] Prof. José António Lopes Department of Nephrology and Renal Transplantation Hospital de Santa Maria, Centro Hospitalar de Lisboa Norte, EPE Av. Prof. Egas Moniz, 1649-035 Lisboa, Portugal Tel. +351961203912 Fax +351217805679 E-mail: jalopes93@hotmail.com Received for publication: 14/02/2013 Accepted: 19/02/2013














