Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.26 no.4 Lisboa out. 2012
Bortezomib in the treatment of refractory kidney graft rejection
Ana Farinha1, Cristina Jorge2, André Weigert2, Margarida Bruges2, Rita Birne2, Patrícia Matias2, Teresa Adragão2, Domingos Machado2
1 Nephrology Department, Centro Hospitalar de Setúbal. Setúbal, Portugal.
2 Division of Transplantation, Nephrology Department, Hospital de Santa Cruz. Carnaxide, Portugal.
ABSTRACT
Background. Bortezomib, a 26S proteasome inhibitor, is a novel treatment for refractory acute rejection. The main mechanism proposed for its action is the induction of apoptosis of mature plasma cells leading to an interruption of donor-specific antibody production, but other properties may also be involved.
Patients and Methods. Four patients presenting with antibody-mediated rejection were treated with bortezomib as rescue therapy. Renal function, proteinuria and anti-HLA antibodies were monitored for 1-3 years.
Results. In three cases, graft function recovery and decreased proteinuria occurred, despite the maintenance of donor-specific antibody levels. However, in one case graft function was lost. Side effects were mostly transient. There were no episodes of opportunistic infection.
Conclusion. Bortezomib may be effective in antibodymediated rejection by different mechanisms than simply through reducing donor-specific antibody levels.
The timing of its introduction and the length of time that donor-specific antibodies have been present may be determinants of its efficacy.
Key-Words: Bortezomib;donor-specific antibody; kidney transplantation; refractory antibody-mediated rejection.
INTRODUCTION
Antibody-mediated rejection (AMR) poses a great challenge in renal transplantation because conventional therapies such as plasmapheresis, intravenous immune globulin (IV Ig), antithymocyte globulin (ATG) or rituximab have limited success in the reversal of this condition1. Their lack of effects on mature plasma cells, and therefore an inability to deplete HLA antibodies known to affect graft survival2, is one of the possible reasons for such unsatisfactory results.
Everly et al. first reported the marked and prolonged reduction in donor-specific anti-human leukocyte antigen antibodies (DSA) observed with the use of bortezomib in the treatment of refractory acute antibody-mediated rejection3. Since then, bortezomib has been tried in several situations in renal transplantation: in AMR, not only as rescue therapy but also as first-line treatment; in chronic humoral rejection; and in desensitisation protocols (for highly sensitised patients)4. However, a variety of protocols and timings of drug introduction have been used and results have been very variable in different reports. Randomised controlled trials have not yet been performed so case reports are important to increase our understanding of the possible benefits of this drug and also to raise new questions about its use in renal transplantation. This article reports our single-centre experience of 4 patients in whom bortezomib was used to treat refractory AMR and reviews other case reports in the literature.
CASE REPORTS
Patient one
This was a 36-year-old Caucasian male with ESRD due to focal segmental glomerulosclerosis (FSGS) in a single kidney who had previously had a kidney transplant for one year before losing the graft because of recurrence of FSGS. He was then on haemodialysis (HD) for 12 years before receiving a second deceased donor kidney graft with 3 HLAantigen mismatches. On this occasion, immunosuppression included ATG induction (10 days), mycophenolate mofetil, sirolimus and prednisone. The nadir serum creatinine (Scr) was 1.2mg/dL. Fourteen months after transplantation (MAT) he developed biopsy-proven acute cellular rejection which was successfully treated with methylprednisolone pulses.
An angiotensin convertor enzyme inhibitor and an angiotensin II receptor antagonist were initiated for proteinuria during the follow-up period. Fifty-seven MAT there was an increase in Scr, with concomitant elevation in class I and II anti-HLA antibodies including DSA B15. Renal allograft biopsy revealed active chronic humoral rejection with double contours and 50% C4d. He was given IV Ig 2g/kg over 48h and Sirolimus was replaced by Tacrolimus. At the 59th MAT, proteinuria and anti-HLA antibodies remained elevated and another biopsy was performed with similar results. Bortezomib was started: the first dose as 1.3mg/m2 was followed by three doses of 0.5mg/m2 because of peripheral neurotoxicity.
Proteinuria was reduced and renal function improved. DSA fell slightly. Since then, two recent further episodes of elevated Scr and proteinuria have been treated with IV Ig 2g/kg over 48h and rituximab 375mg/m2, because of the neurotoxicity with bortezomib. Three years after therapy, the allograft continues to function with a Scr of 2.5 mg/dL (Figure 1).
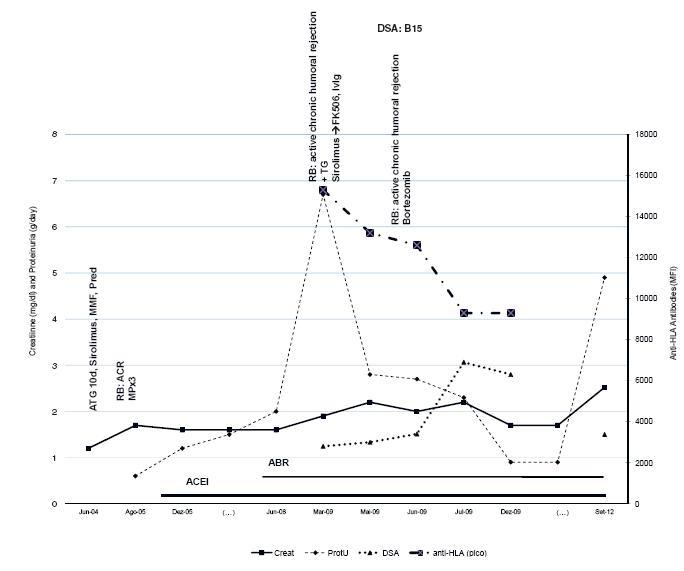
Figure 1
Patient 1 evolution and treatment timing. Serum creatinine, proteinuria, anti -HLA levels (highest level) and DSA expressed in mean fluorescence intensity (MFI), are shown. The time is presented with gaps represented as (...). ABR – angiotensin II blocker receptor; ACEI – angiotensin conversion enzyme inhibitor; ATG – anti -T -lymphocyte immunoglobulin; Pred – prednisolone; TG – transplant glomerulopathy; RB – renal biopsy.
Patient two
This was a 21-year-old Caucasian female with ESRD due to chronic pyelonephritis secondary to vesicoureteric reflux. She had previously had a kidney transplant for 9 months after which she had suffered irreversible acute cellular rejection. She received a second deceased-donor kidney transplant with 4 HLA antigen-mismatches. Cold ischaemia time was 22 hours and 33 minutes. She previously had panelreactive antibody (PRA) 0% and a negative AHA-CDC crossmatch (CXM), so immunosuppression consisted of basiliximab induction, mycophenolate mofetil, tacrolimus and prednisone. However, when the immunologic results immediately prior to transplantation became available, it was discovered that she had high levels of anti-HLA antibodies class I (maximum 17995 MFI) and class II (maximum 11890 MFI) including 3 DSA (B27 11382 MFI, B8 7665 MFI, A11 5657 MFI), and a positive T Lymphocyte CXM by flow cytometry.
On the 5th day post-transplantation, she started IV Ig 2g/kg over 48h and rituximab 375mg/m2. Serum creatinine fell to a nadir of 1.24mg/dL but DSA continued to increase (14000 MFI). Four MAT, Scr increased from 1.3mg/dL to 1.9mg/dL, with no proteinuria but with further elevation of DSA. Renal allograft biopsy showed acute humoral rejection. She again received IV Ig 2g/kg and rituximab 375mg/m2.
This therapy was repeated on two more occasions (4th and 9th MAT). Scr fell to 1.5mg/dL, but DSA remained elevated. In the 10th MAT, de novo proteinuria (1.5g/day) developed. In the 12th MAT, proteinuria rose to 3.6g/day and Scr to 2.3mg/dL, so a second renal allograft biopsy was performed. It showed active chronic humoral rejection. A further course of IV Ig 2g/kg and rituximab 375mg/m2 was given but, as renal laboratory results did not improve and DSA increased (10000 MFI), we decided to give 4 doses of bortezomib 1.3mg/m2 every four days.
Scr fell to 1.6mg/dL, proteinuria to 1.4g/dL and DSA to 6000 MFI. She complained of lower limb pain that improved after the last bortezomib dose. In the 13th MAT, a new increase in SCr (4.6mg/dL) and proteinuria (4.6g/day) was observed. A third renal allograft biopsy confirmed active chronic humoral rejection and acute cellular rejection (Banff IA). By this time she required haemodialysis. A second cycle of bortezomib 4 doses every four days at reduced dosage (1mg/m2), was given after plasmapheresis (3 daily sessions followed by 3 sessions on alternate days) and 3 doses of methylprednisolone 500mg. Scr fell to 1.8mg/dL and proteinuria remained stable but DSA rose to 9000 MFI. She became independent of dialysis for a short period. Then, in the 14th MAT, a new rise
in Scr and proteinuria prompted us to give a third cycle of bortezomib 0.5mg/m2 with rituximab 375mg/m2. However, she remained dependent on haemodialysis and a graft nephrectomy was performed (Figure 2).
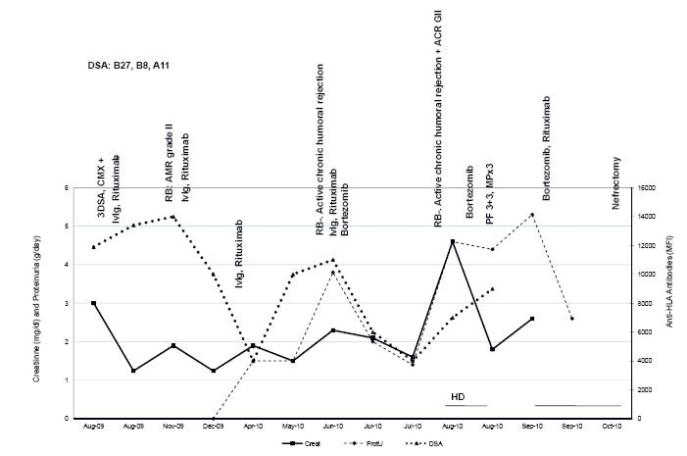
Figure 2
Patient 2 evolution and treatment timing. There are represented creatinine, proteinuria and DSA (highest level) expressed in mean fluorescence intensity (MFI). HD – haemodialysis; MP – methylprednisolone; PF – plasmapheresis; RB – renal biopsy.
Patient three
This was a 25-year-old Caucasian female on HD for ESRD due to lupus nephritis who received a kidney from her mother with whom she had 3 HLAantigen mismatches. Immunosuppression included basiliximab induction, mycophenolate mofetil, tacrolimus and prednisone. Forty-four MAT, her Scr increased from 0.9 to 1.5mg/dL and de novo proteinuria (1.6 g/day) was detected. High serum levels of class I and class II anti-HLA antibodies were detected (peak of 14579 MFI and 11831 MFI respectively), including a DSA of 2019 MFI. A T-lymphocyte CXM by flow cytometry was positive. A renal allograft biopsy demonstrated active chronic humoral rejection so IV Ig 2g/kg over 48h and rituximab 375 mg/m2 were given. There was no improvement in her renal function so she received further IV Ig 2g/kg and 4 sessions of plasmapheresis between 45th and 47th MAT. Her Scr was 1.3 mg/dL until the 69th MAT when it increased to 1.5 mg/dL. Once more, T lymphocyte CXM by flow cytometry was positive. IV Ig 2g/kg and rituximab 375mg/m2 were given again. In the 75th MAT her Scr increased to 2.1 mg/dL and anti-HLA antibodies to 13700 MFI. A further renal allograft biopsy showed active chronic humoral rejection with diffuse positive C4d. Bortezomib 1.3mg/m2, four doses every four days, was given along with rituximab 375mg/m2. Scr fell to 1.9mg/dL after treatment and remained stable. Renal function has been maintained over the next 18 months, though another cycle of IV Ig 2g/kg and rituximab 375 mg/m2 has been given. (Figure 3).
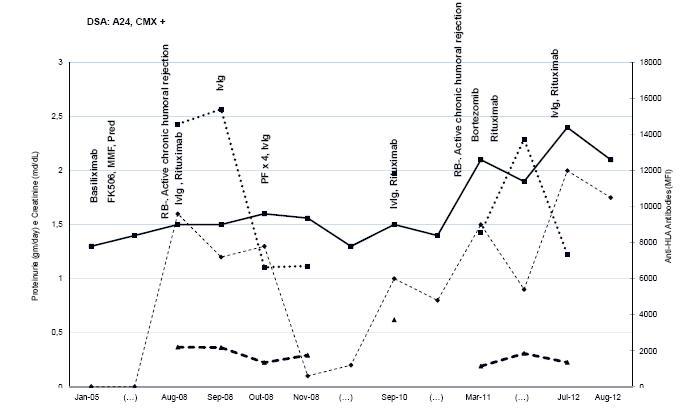
Figure 3
Patient 3 evolution and treatment timing. Creatinine, proteinuria, DSA and anti-HLA levels are expressed in MFI. The time is presented with gaps represented as (...). PF – plasmapheresis; RB – renal biopsy.
Patient four
This was a 42-year-old Caucasian male with ESRD due to medullar cystic disease who had previously had a kidney transplant for 3 years before losing the graft because of chronic allograft nephropathy.
He then remained on HD for 14 years, after which he received a second deceased-donor kidney graft with 4 HLA-antigen mismatches. Cold ischaemia time was 17 hours. He had serum anti-HLA antibodies class I and II (peak 5917 and 10901 MFI respectively) but no DSA. AHA-CDC CMX was negative. Immunosuppression included ATG (7 days), IV Ig 2g/kg over 48h and rituximab 375mg/m2 (2 days) induction, and maintenance with mycophenolate mofetil, tacrolimus and prednisone. At 13th MAT, SCr increased from 1.6mg/dL to 2.5mg/dL, so anti-HLA testing was repeated and a DSA was identified. A renal allograft biopsy showed acute humoral rejection grade II. He received IV Ig 2g/kg over 48h and rituximab 375 mg/m2 and his Scr returned to 1.5mg/dL. Thirty-two MAT a new rise in Scr was observed (1.7 mg/dL), with proteinuria 0.5g/day. Anti-HLA antibodies increased to 10715 MFI, with a DSA of 8257 MFI. A second renal allograft biopsy confirmed acute humoral rejection and allograft glomerulopathy. Bortezomib 1.3mg/m2, three doses every four days, was given. The patient complained of transient diarrhoea and symptoms of neuropathy (pain in lower limbs). Scr fell to 1.5mg/dL and proteinuria to 460mg/day without any further treatment. He remains stable after 18 months of follow up (Figure 4).
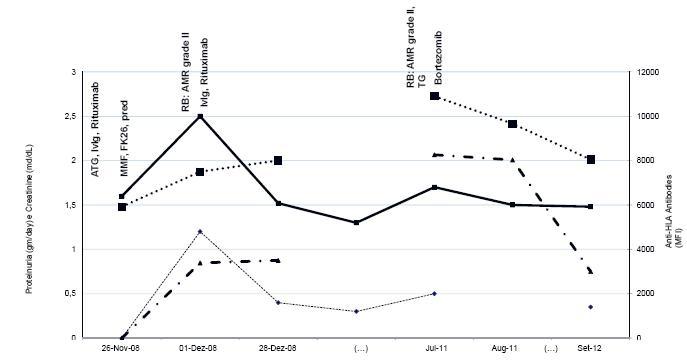
Figure 4
Patient 4 evolution and treatment timing. Creatinine, proteinuria, DSA and anti-HLA levels are expressed in MFI. The time is presented with few gaps represented as (...). AMR – antibody mediated rejection; ATG – anti -T -lymphocyte immunoglobulin; TG – transplant glomerulopathy; RB – renal biopsy.
DISCUSSION
It has been difficult to evaluate and establish the role of bortezomib in the treatment of antibody mediated rejection in renal transplantation because different regimens have been used in a limited number of case reports, with variable results.
We present one case of failed treatment with bortezomib and three cases where graft function recovered, with simultaneous reduction of proteinuria. This improvement was not accompanied by DSA reduction. Although most reports have considered bortezomibs action on plasma cells to reduce anti- HLA production to be of most significance, several other mechanisms may be responsible for the good results that have been reported. Everly et al.3 highlighted the importance of reduction in DSA levels, but they also proposed other mechanisms including induction of apoptosis in activated T cells, T-cell depletion, NF-kB inhibition, reduced major histocompatibility complex class I expression, decreased Th1 responses, dendritic cell function inhibition (reduced costimulatory molecule expression, reduced cytokine production and apoptosis) and inhibition of IL-6 production by bone marrow stromal cells leading to apoptosis at various stages in B-cell maturation3. Perry et al.5 in their important contribution to the understanding of bortezomib's effects, proposed similar mechanisms to explain their results. Bortezomib's pleiotropic effects have also been highlighted in other studies6-9.
Another issue that must be emphasised is the importance of associated therapies. Some studies have shown that bortezomib alone may not decrease DSA levels4. Plasmapheresis has been proposed to be critical in this setting. Walsh et al.8 supported the use of plasmapheresis, not only for its action in removing previously secreted antibodies, but also because removal of circulating antibody results in increased antibody production or metabolic demands on B cells, memory B cells and plasma cells, thereby potentially enhancing sensitivity to proteasome inhibition8.
When Leyva et al.10 presented their experience, they also concluded that bortezomib should be given in association with plasmapheresis because of poor results in patients who had not received this adjuvant therapy.
Other reports suggest other adjuvant therapies. Walsh et al. propose the use of rituximab on the assumption that early acute AMR involves active generation of new plasma cells and plasmablasts from existing memory B-cell populations. In this context, rituximab should potentiate bortezomib's effects by reducing plasma cell generation from the memory B-cell population8. Other series have also shown better results with concomitant use of Rituximab8,11,12.
Finally, some studies have emphasised the importance of concomitant steroids because of synergistic proapoptotic effect in normal plasma cells13,14.
The anti-HLA itself may be a determinant of the results of treatment. Sberro-Soussan et al., who reported several unsuccessfully-treated cases13,15, proposed lack of activity against DSA because of a long period of DSA stability before the use of bortezomib.
The activity of bortezomib probably correlates with the speed of proliferation of memory B-cell and plasma cell populations11: the faster that plasma cells are proliferating (reflected by a higher level of anti-HLA), the more susceptible they are likely to be to therapy. The number of specificities and timing of DSA formation may be important11. Type and pattern of DSA formation appear to influence resistance to bortezomib13 since evidence has been published that DR52 is refractory to treatment with plasmapheresis and IV Ig16 and may also be resistant to bortezomib therapy.
The timing of antibody rejection may be important: it has been proposed that results are better in early versus late AMR17 and several authors have reported good results in early AMR8,12,18,19, in contrast to suboptimal results in late AMR17,20,21. This could explain why bortezomib seems to be less effective in desensitisation protocols4.
The late timing of use of bortezomib may be expressed clinically by prolonged elevation of Scr and histologically by transplant glomerulopathy22.
Fletcher et al.23 proposed that treatment is likely to be more effective before the onset of significant renal dysfunction (Scr<3mg/dL) or proteinuria (<1g/day)14, and a similar observation has been made by Sberro et al.13 and other authors18,24. Few studies have investigated the long-term histological impact of bortezomib therapy25.
Our unsuccessful case corroborates the observations that established histological damage caused by multiple events, and expressed clinically by elevated serum creatinine and proteinuria, are irreversible even with bortezomib use; for this patient, we started bortezomib only after almost 10 months and several cycles of preceding conventional therapy. It may also be representative of the finding that anti-HLA reduction seems to be greater in de novo DSA than in pre-formed ones4,24,26. In spite of these considerations, our first and third patients seemed to respond to therapy. Indeed, although bortezomib was introduced at a late stage (glomerulopathy lesions were already present) their renal function improved. This occurred even with the persistence of high anti-HLA levels.
A number of questions about the use of botezomib in the treatment of kidney transplant rejection still remain to be answered. When exactly is bortezomib indicated? What is the best regimen: one or more cycles, with or without adjunctive therapy? How long should we wait for DSA reduction? Does bortezomib prevent progression to chronicity? What are the longterm benefits? How can we predict response to therapy? And what about adverse outcomes? While we wait for definitive conclusions that will only be possible with a randomised controlled trial, we believe that our series may be useful to other physicians who deal with AMR.
References
1. Lucas JG, Co JP, Nwaogwugwu UT, et al. Antibody-mediated rejection in kidney transplantation: an update Expert Opin Pharmacother 2011;12:579-592 [ Links ]
2. Everly MJ, Everly JJ, Susskind B, et al. Proteasome Inhibition Reduces Donor-Specific Antibody Levels. Transplant Proc 2009;41:105-107 [ Links ]
3. Everly MJ, Everly JJ, Susskind B, et al. Bortezomib provides effective therapy for antibody-and cell-mediated acute rejection. Transplantation 2008; 86:1754-1761 [ Links ]
4. Everly MJ, A summary of Bortezomib use in transplantation across 29 centers. Clin Transpl 2009;323-337 [ Links ]
5. Perry DK, Burns JM, Pollinger HS, et al. Proteasome inhibition causes apoptosis of normal human plasma cells preventing alloantibody production. Am J Transplant 2009;9:201-209 [ Links ]
6. Djamali A, Muth BL, Torrealba J, et al. Bortezomib as rescue therapy for hyperacute and multi-drug resistant mixed acute rejection after kidney transplantation. Clin Transpl 2009:485-490 [ Links ]
7. Everly MJ, Everly JJ, Susskind B, et al. Proteasome inhibition reduces donor-specific antibody levels. Transplant Proc 2009;41:105-107 [ Links ]
8. Walsh RC, Everly JJ, Brailey P. Proteasome inhibitor-based primary therapy for antibodymediated renal allograft rejection. Transplantation 2010;89:277-284 [ Links ]
9. Lemy A, Toungouz M, Abramowicz D. Bortezomib: a new player in pre- and posttransplant desensitization? Nephrol Dial Transplant 2010;25:3480-3489 [ Links ]
10. Levya S, Marino-Vasquez LA, Reyes-Loaeza JA, et al. Bortezomib for antibody mediated Rejection treatment: experience at the Instituto Nacional de Ciencias Médicasy Nutrición Salvador Zubiran in Mexico City. Clin Transpl 2009:369-375 [ Links ]
11. Manitpisitkul W, Wilson NS, Philosophe B, et al. Rescue Therapy for acute antibody mediated rejection with a protease inhibitor after kidney transplantation. Clin Transpl 2010:421-428 [ Links ]
12. Lashmann N, Schutz M, Budde K, et al. Anti-humoral rejection therapy by proteasome inhibitor bortezomib: a case series. Clin Transpl 2009:351-358 [ Links ]
13. Sberro-Soussan R, Zuber J, Suberbielle-Boissel C, et al. Bortezomib alone fails to decrease donor specific anti-HLA antibodies: even after one year post-treatment. Clin Transpl 2010:409-414 [ Links ]
14. Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed refractory myeloma. N Engl J Med 2003;348:2609-2617 [ Links ]
15. Sberro-Soussan R, Zuber J, Suberbielle-Boissel C, Legendre C. Bortezomib alone fails to decrease donor specific anti-HLA antibodies: 4 case reports. Clin Transpl 2009:433-438 [ Links ]
16. Zachary AA, Montgomery RA, Leffell MS. Factors associated with and predictive of persistence of donor specific antibody after treatment with plasmapheresis and intravenous immunoglobulin. Hum Immunol 2005;66:364-370 [ Links ]
17. Walsh RC, Alloway RR, Girnita AL, et al. Proteosome inhibitor therapy for antibodymediated rejection. Kidney Int 2012;81:1067-1074 [ Links ]
18. Kaneku H. Annual literature review of donor-specific HLA antibodies after organ transplantation. Clin Transpl 2011:311-318 [ Links ]
19. Woodle ES, Alloway RR, Girnita A. Proteosome inhibitor treatment of antibody-mediated allograft rejection. Curr Opin Organ Transplant 2011;16:434-8 [ Links ]
20. Al Meshari K, Pall A, Elgamal H, et al. Bortezomib as an adjuvant to conventional therapy in the treatment of antibody mediated rejection (AMR): the full spectrum. Clin Transpl 2010:383-390 [ Links ]
21. Guthoff M, Schmid-Horch B, Weisel KC, et al. Proteosome inhibition by bortezomib: effect on HLA-antibody levels and specificity in sensitized patients awaiting renal allograft transplantation. Transpl Immunol 2012;26:171-175 [ Links ]
22. Schwaiger E, Regele H, Wahrmann M, et al. Bortezomib or the treatment of chronic antibody-mediated kidney allograft rejection: a case report. Clin Transpl 2010;391-396 [ Links ]
23. Wong W, Lee RA, Saidman SL, et al. Bortezomib in Kidney transplant recipients with antibody mediated rejection: three case reports. Clin Transpl 2009;401-405 [ Links ]
24. Pavlakis M. case presentations on two patients who received bortezomib for antibody mediated rejection at BIDMC. Clin Transpl 2009;343-345 [ Links ]
25. Hardinger KL, Alford K, Murillo D. Bortezomib as rescue therapy for antibody mediated rejection: a single-center experience. Clin Transpl 2010;429-436 [ Links ]
26. Fairfax KA, Kallies A, Nutt SL, et al. Plasma cell development: from B-cell subsets to long term survival niches. Sem Immunol 2008;20:49-58 [ Links ]
Dr Ana Farinha
Av. D. João II, nº12, 7º D
2910-548 Setúbal, Portugal
E-mail: alpfarinha@yahoo.com.br
Conflict of interest. None declared.
Received for publication: 09/11/2012
Accepted in revised form: 20/11/2012














