Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.26 no.4 Lisboa out. 2012
Arteriovenous fistula surveillance: everyones responsibility
Martin Anek Feddersen BA, MBBS and Simon David Roger MD, FRACP
Department of Renal Medicine, Gosford Hospital. Gosford, NSW, Australia.
ABSTRACT
The arteriovenous fistula, as opposed to arteriovenous grafts and central venous dialysis catheters, remains the dialysis access of choice for haemodialysis patients, due to its superior long -term patency, low incidence of stenosis, thrombosis and infection.
The basic tenant of vascular access monitoring and surveillance is that stenosis develops over a variable amount of time in a majority of arteriovenous fistula, and if detected and corrected, inadequate dialysis can be prevented, maturation can be facilitated and thrombosis and access loss can be avoided. Large randomised controlled trials are lacking to clearly identify the ideal surveillance strategies and benefits of surveillance, often only supported by observational studies and small single-centre randomised controlled trials. Fistula thrombosis is often used as an endpoint in trials looking at the beneficial effects of surveillance, although this occurs relatively infrequently in native arteriovenous fistula, and therefore other markers are required to define a dysfunctional fistula.
Cost-effective ways to improve outcomes in these types of vascular accesses involves formalised training of staff and other healthcare professionals to better identify dysfunctional fistulas by physical examination, with the addition of surveillance studies to support these findings and pre -emptive intervention when stenosis is found. The costs invested in establishing multidisciplinary programmes to facilitate the care of these patients will likely reduce long–term resource utilisation in a growing population of dialysis patients worldwide. In this review, we examine the physiology of a dysfunctional fistula and evaluate available studies in the surveillance of arteriovenous fistulas. In addition, the importance of creating secondary arteriovenous fistula and how healthcare systems need to invest in improving the care of haemodialysis vascular access will be outlined.
Key-Words: Arteriovenous fistula; dysfunctional fistula; haemodialysis; monitoring; stenosis; surveillance.
INTRODUCTION
The incidence of end-stage renal disease (ESRD) and the need for renal replacement therapies (RRT) continues to rise in all national registries1-4. The lack of donor organs available for renal transplantation has left a large cohort of patients reliant on dialysis as a long -term treatment option.
Dialysis access placement and maintenance in the haemodialysis population is paramount to the short - and long - term survival of these patients5-7. Complications of vascular access contribute a large overall cost burden, with access-related complications associated with 15 -24% of hospital admissions in ESRD patients undergoing haemodialysis8,9.
Arteriovenous fistula (AVF) failure requires placement of a central venous dialysis catheter and is associated with significant morbidity and mortality10.
AVFs are the access of choice in haemodialysis patients, due to their superior long-term patency and low incidence of stenosis, thrombosis and infection11-13.
Reduction of blood flow due to stenosis can lead to reduction in dialysis dosage and thrombosis requiring endovascular or surgical revision of the AVF14.
The vast majority of clotted fistulas have an underlying stenosis15. AVF surveillance with various noninvasive methods have been studied to detect subclinical stenosis, as well as the early management of these lesions, but mixed results have been reported regarding the clinical efficacy of this approach, and much controversy still exists16.
There are many reasons why this has occurred, including the heterogeneity of surveillance methods available, the definition of what a clinically relevant stenosis is, as well as the manner in which these lesions are treated within institutions. Endpoints of surveillance studies have also been largely based on thrombosis, which occurs relatively seldom in the natural history of an AVF and therefore have left many of these studies underpowered11.
A much more relevant marker of success of a surveillance programme needs to be identified, which may include traditional dialysis adequacy targets e.g. Kt/V and/or URR as well as fistula blood flow and other clinical markers. This article will describe briefly the physiology of inflow and outflow stenosis in AVF and review relevant randomised control trials of AVF monitoring and surveillance. We will also discuss the importance of secondary AVF and how healthcare systems need to invest in improving the care of haemodialysis vascular access for the future.
THE DYSFUNCTIONAL ARTERIOVENOUS FISTULA
It is difficult to accurately define the dysfunctional fistula. Efforts to standardise definitions and to create a network to promote collaboration in this area will make this more efficient in the future17. Commonly accepted clinical signs may include variations in thrill/bruit, difficult cannulation, the inability to support a reasonable dialysis pump speed, excessive recirculation, reduced flow on surveillance testing, reduced dialysis adequacy targets e.g. Kt/V and URR and ultimately thrombosis.
Unlike an arteriovenous graft (AVG) with one inflow and one outflow, forearm AVF (commonly radio-cephalic) have one inflow vessel, but multiple outflow vessels (Fig.1). They also have the advantage of being superficial, accessible, rarely developing steal syndrome or becoming giant fistulas. This differs from upper arm AVF (more often brachio-cephalic than brachio-basilic) which are physiologically more similar to an AVG, given the lack of outflow vessels (Fig. 2). A forearm AVF is therefore a low -resistance, low -pressure circuit able to stay patent with low blood flows with inflow stenosis occurring most commonly, unlike upper arm AVF and an AVG which are often affected by outflow stenosis and traditionally more difficult to treat.
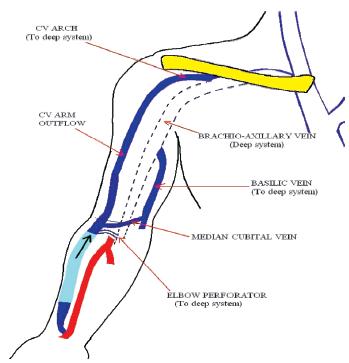
Figure 1
Radio-cephalic arteriovenous fistula with one inflow vessel and multiple outflow Vessels
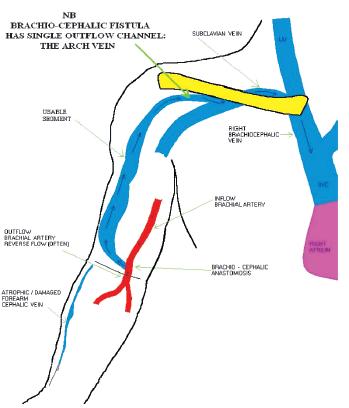
Figure 2
Upper arm arteriovenous fistula with single outflow vessel
With the development of access stenosis, the narrowed segment changes into a high-pressure segment with a subsequent reduction in fistula blood flow, the effect of which varies according to the severity, position, number of stenosis, presence of collaterals and type of access e.g. upper or lower arm AVF. The effect of an inflow stenosis results in reduced blood flow after the stenotic segment with high negative arterial pressures and an increased risk of recirculation (Fig. 3). Outflow stenosis also causes a reduced blood flow, with maintained pressures in the fistula until the stenosis, sometimes with high venous return pressures and recirculation, depending on the location of the lesion (Fig. 4).
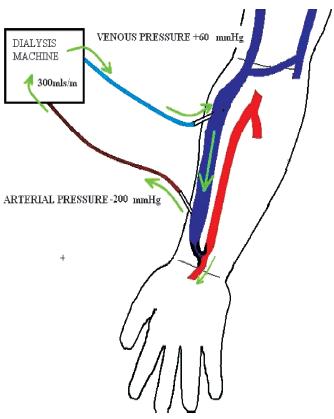
Reduced flow after stenosis with a rapid drop in pressure causing sucking of arterial line with increased arterial pressure. A reduction in blood flow results in a higher risk of recirculation
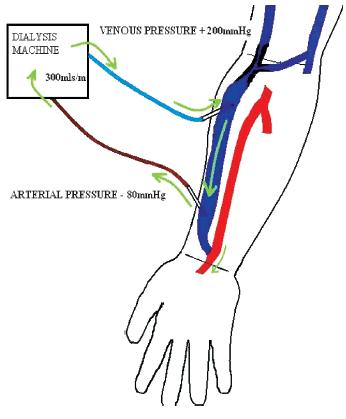
Figure 4
Reduction of flow with maintained pressure within fistula until stenosis. High venous return pressure with some recirculation also possible
ARTERIOVENOUS FISTULA MONITORING AND SURVEILLANCE
The basic tenant of vascular access monitoring and surveillance is that a stenosis develops over a variable amount of time in a majority of AVF, and if detected and corrected, inadequate dialysis can be prevented, maturation can be facilitated and thrombosis and access loss can be avoided18.
The establishment of a functioning fistula begins early in the process of impending ESRD, with a multidisciplinary approach. Preservation of veins, education of patients and other medical staff and the identification of the best vessels to create an AVF all play an important part in healthy vascular access and subsequently adequate dialysis19 -21. The benefits of a vascular access coordinator in guiding this process and improving outcomes in these patients have been clearly documented20,22,23. The vast majority of research into haemodialysis access comes from the United States where AVG are still highly prevalent, and is therefore biased towards measuring the success of surveillance in this type of access. AVG have a completely different natural history to that of an AVF.
The frequency of AVF thrombosis, a common endpoint for most studies looking at the benefit of surveillance, have difficulty in demonstrating a benefit due to the infrequent occurrence of thrombosis, with an estimated frequency of 0.25 episodes/patient/yearor less11,18,24,25. This is due to the unique physiology of native fistulas and their ability to maintain patency at very low blood flow rates.
What adds more confusion is how to define a physiologically significant stenosis when one is located.
Fartash described defining percentage stenosis with adjacent vessels is often inaccurate26 (Fig. 5). In other words, a stenotic area can have a percent stenosis that ranges from 0% to +75% depending on which part of the fistula it is compared against and is therefore a highly inaccurate way to define stenosis. In this study a minimal luminal diameter of 2.7mm had a sensitivity of 90% and specificity of 80% with an area under the receiver operator curve of 90% in distinguishing functional from a dysfunctional radio -cephalic fistula when using greyscale or colour ultrasound.
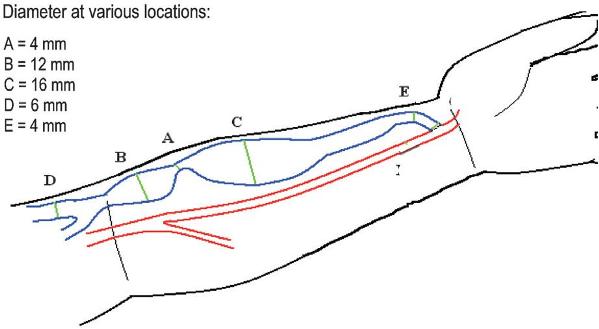
Figure 5
Shown here is a typical radio-cephalic fistula with a wide variation of blood vessel diameter. The current definition of stenosis is inaccurate as it expresses stenosis as a percentage value as compared to an adjacent vessel. When comparing stenosis A to other parts of the fistula circuit there is a wide variation in percent value 0% to + 75%. A more accurate measure of stenosis may be achieved by using a direct measure of diameter (mm)
What is important to note though is that in this study a dysfunctional fistula was defined according to abnormal dynamic dialysis pressures, now known not be an inaccurate measure of fistula function, with a point made that fistula blood flow (Qa) studies, the current gold standard, were not available for patients at the time of the study. Further research needs to be done examining the association of stenosis diameter and its effects on blood flow through AVF.
Confusion lies in that observational studies are not in line with randomised controlled trials which are often hampered by small numbers, insufficient power and short follow-up. Currently evidence suggests that screening is beneficial with AVF but not AVG27. This is due to the large range of fistula blood flow over which an AVG can thrombose, and the difficulty in defining a cut -off for diagnostic investigations to correct stenosis and prevent thrombosis.
Monitoring
The cornerstone of every access surveillance programme is regular monitoring by means of physical examination28-30. With adequate training nephrologists and dialysis staff are able to screen fistulas and identify dysfunctional fistulas that may require referral for more definitive investigations, e.g. Doppler ultrasound or angiography31. A paucity of studies is available to assess the clinical accuracy of monitoring and conflicting evidence exists. Maya et al. in their prospective two-year study of 543 fistulograms showed a relatively poor correlation of identifying a significant (>50%) stenosis on angiography, with the likelihood of an abnormal physical examination, which were lower in AVF than in AVG (39.4% vs. 68.7%; P<0.001)32. Diagnostic investigations were initiated by nursing staff and may highlight, not only the importance of proper training, but also the need for other indicators of fistula dysfunction to be used for more appropriate referral for more invasive testing.
Physical examination with intra-access pressure measurements for the detection of AVF stenosis, (confirmed by Doppler ultrasound) was compared by Campos. The accuracy of physical examination and intra-access pressure were 88% and 71% with sensitivity, specificity, positive predictive value and negative predictive being higher with PE33. Although clinical assessment was superior in this study, when observing these results, one has to take into account the inherent deficiency of pressure measurement in a low-pressure circuit such as an AVF.
Another study by Asif showed a strong correlation between physical examination in the diagnosis of outflow and inflow stenosis with sensitivity and specificity of the former 92% and 86% and the latter 85% and 71% respectively34. The physical examination included inspection (arm, shoulder, breast, neck and face oedema and the presence of collaterals), palpation (pulse, thrill, pulse augmentation and arm elevation) and auscultation in a systematic manner. Unlike the previous study this may reflect the variation in skill levels available and again underlines the importance of training in this area.
The physical examination should also extend to patients with chronic renal disease Stage IV or V who are being prepared for haemodialysis to screen those AVF which have failed to mature adequately.
Endovascular techniques used in managing late fistula failure are now being used to assist in fistula maturation, again reducing the requirement of tunneled vascular catheters35 (Fig. 6). Early identification of a non-maturing fistula allows for maturation to be facilitated by fistuloplasty, with or without stent insertion, or obliteration of venous collaterals, now routinely practiced in many institutions36-40.
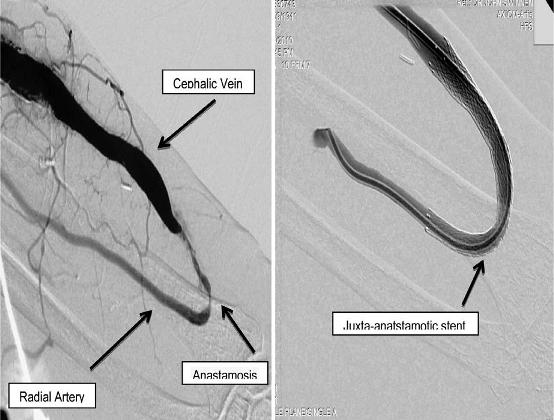
Figure 6
Pre and post treatment of a radio-cephalic arteriovenous fistula with a juxtaanastomotic stenosis via endovascular stent insertion. This technique is used both in assisting fistula maturation as well as treating late fistula stenosis
Surveillance
Surveillance involves the use of specialised tests done by trained staff to identify a dysfunctional fistula by measuring variations in pressure, blood flow and/or direct visualisation of the fistula circuit.
Surveillance methods have reduced the variability of findings seen in clinical monitoring by standardizing the assessment of fistulas, but with added expenses.
Blood-Flow Surveillance
Blood flow can be either directly or indirectly measured.
The modality chosen depends largely on established practices and resources available to each unit, but guidelines such as the National Kidney Foundation – Dialysis Outcomes Quality Initiative (NFK -KDOQI) 2006 Practice Guidelines give some direction11. Criticism of these guidelines often reflect the lack of evidence available to make strong recommendations and many questions still remain unanswered regarding optimal monitoring and surveillance strategies41.
Indirect measurements include an ever-expanding selection with ultrasound dilution technique recognized as the current gold standard42. Other techniques include the glucose pump test43, ionic dialysance44, differential conductivity45 and transcutaneous flow rate measurement46. Direct measurement of blood flow includes MRA and Doppler ultrasound which also has the advantage of giving anatomical information, such as the location and grade of the stenosis and can thus assist in planning interventions, but the disadvantage of being more expensive and time consuming (Fig. 7). As recommended by KDOQI guidelines surveillance should identify trends suggestive of a dysfunctional fistula and therefore serial measurements need to be done on patients, given the inherent variability in needle placement. The threshold for detecting access stenosis varies with type and location of the vascular access.
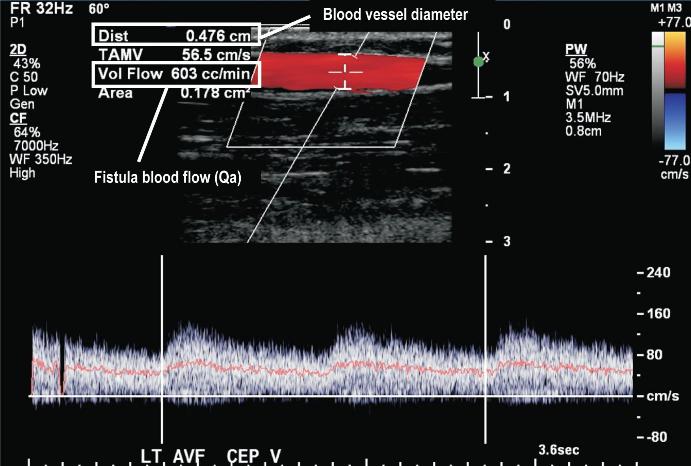
Doppler ultrasound is an excellent tool for triaging patients for more invasive investigations allowing interventionalists to plan procedures, by measuring vessel diameter and calculating fistula blood flows (Qa)
So far only a few randomised controlled trials have looked at the benefits of surveillance using vascular access flow (Qa) to detect early stenosis and the subsequent reduction of thrombosis rates47,48. Polkinghorne looked at the detection of access stenosis by randomly assigning 137 patients to either receive AVF surveillance using usual treatment (control group) or usual treatment plus Qa via ultrasound dilution (Qa surveillance group).
Usual treatment consisted of referral for fistulography if one of the following occurred: excessive bleeding from venepuncture site, reduction in URR, changes in dynamic venous pressure and decrease in blood pump flow (Qb). Patients in the Qa surveillance arm were referred for a fistulogram if Qa < 500ml/min or it fell by > 20% once the access flow was <1000ml/min. Primary endpoint was stenosis of ≥ 50%. Patients in the Qa surveillance group were twice as likely to have a stenosis detected compared with the control hazard ratio (HR) confidence interval (CI) group (2.27, 95% 0.85 -5.98, P = 0.09), with a trend for a significant stenosis to be detected earlier in the Qa surveillance group (P = 0.09, log rank test).
However, using the Qa results alone prior to angiography, showed the area under the receiver operating curve demonstrating at best, a moderate predictive value of > 50% AVF stenosis (0.78, 95% CI 0.63-0.94, P = 0.006). It was concluded that Qa surveillance doubled the detection of angiographic AVF stenosis, but these results were not statistically significant and further larger trials needed to be undertaken to clarify if a reduction in thrombosis and an increase in AVF survival could be achieved with Qa surveillance47.
An older study by Sands evaluated thrombosis rates, but included both AVF and AVG with the latter having a significantly higher frequency of thrombosis than the former48. One hundred and three patients were randomised (68 AVF, 35 AVG) for a mean followup of 197 days to monthly monitoring with Qa and static venous pressure or no monitoring. Patients with access flow <750 ml/min or with static venous pressure ≥ 0.5 were referred for angiography and angioplasty of stenotic lesions ≥ 50%. Six of sixtytwo (9.7%) of monthly monitored patients developed access thrombosis vs. nine of forty -one (22%) of control patients (p<0.05). It was concluded that monthly access flow measurements would ensure a lower incidence of thrombosis and decrease the cost of access maintenance.
Another study looking at forearm AVF compared ultrasound dilution technique and colour Doppler ultrasound and calculated the cut-off value for the prediction of stenosis was 465ml/min for the former and 390 ml/min for the latter. Good to note was that receiver operator curves showed similar performance between both techniques with areas under the curve averaging 0.79 (95% [CI], 0.66 to 0.91) for ultrasound dilution technique and 0.80 (95% CI, 0.65 to 0.94) for colour Doppler ultrasound, meaning that either technique can be used to calculate blood flow rate with the advantage of anatomical information when using ultrasound49 (Fig.7).
Pressure Surveillance
Given the physiology of an AVF when compared to an AVG, intra -access pressure tends to be variable and unpredictable, due to the presence of draining collaterals. Measurement of intra -access pressure involves observing pressure gradients between the artery and vein with a great variability in measurement depending on the site at which the pressure is measured and position of the fistula relative to the heart50,51. These pressures can be static or dynamic depending on if they are measured while the patient is receiving dialysis at the time of measurement or not.
In general, both static and dynamic venous pressures have a low positive predictive value at detecting stenosis and are more suited to for an AVG, with a single point of outflow11,52 -54. It is important to note that although access pressures may not be ideal in AVF surveillance, it can still be used in conjunction with other mentioned surveillance techniques to help identify a dysfunctional fistula, but not as a sole marker of stenosis.
Recirculation
Recirculation is often thought of as a late manifestation of AVF dysfunction, but may still be a useful tool in assessing inflow or juxta-anastomotic stenosis (Fig.3). Radio -cephalic AVF are the most common form of native vascular access with inflow problems due to juxta -anatstomotic stenosis, thought to occur following manipulation of the cephalic vein as it is being attached to the radial artery, occurring most frequently both in the setting of fistulas failing to mature and late fistula stenosis. The utility of recirculation in these types of accesses may be understated and it may be a sensitive marker for inflow stenosis in forearm fistulas. AVF recirculation has two components, vascular access recirculation and cardiopulmonary recirculation.
The former occurs when blood returning to the access is drawn back into the arterial line without returning to the patient, as a result of blood pump being greater than fistula blood flow (Qa), limiting effectiveness of dialysis by preventing equilibration of dialysed and non -dialysed blood. Several determinants of vascular access recirculation exist including access flow to pump speed, distance and positioning of needle tips, orientation of lines, access stenosis and type.
Cardiopulmonary recirculation involves dialysed blood returning to the heart via the venous system directed back to the vascular access without reaching the peripheries. In patients with cardiac disease and/or poor perfusion the peripheral circulation is poor, limiting the equilibration of dialysed blood with poorly perfused tissue. Cardiopulmonary recirculation often remains undetected for many years as it is often neglected and may be significant in some patients.
Several different methods are used to assess recirculation including urea, glucose and thermodilution based methods, with the ultrasound dilution method currently considered the gold standard, as it does not include cardiopulmonary recirculation55,56. NFK –KDOQI Practice Guidelines suggest a recirculation value of >10% to warrant further investigations using urea based methods11. Non-urea based recirculation studies using ultrasound dilution technique are significantly more sensitive with values of >5% having a high sensitivity and specificity for diagnosing stenosis57.
Long -term access patency
Long-term patency of stenotic AVF has not been extensively investigated with the main focus being thrombosis due to stenosis, but several studies have tried to explore this issue25,58 -60. A randomised controlled study by Tessitore identified 79 AVF with angiographically proven stenosis of > 50% and randomised one group to pre-emptive treatment and the other group to intervention done in response to a decline in dialysis dose or thrombosis25.
Referral for angiography was made when recirculation was >5%, pump blood flow (Qb) decrease >30ml/min, Qa <750ml/min, or there was a >25% reduction in Qa. These parameters were described in a previous study to have an excellent diagnostic accuracy of 95% sensitivity and 86% specificity in diagnosing stenosis57. Fistulas were further identified as functional or failing depending on their Qa of 350ml/min higher or lower.
In conclusion, over five years blood flow surveillance and pre -emptive repair prolonged the functional life of a mature AV fistula with a loss rate of 15.6% per year in the control and 5.1% per year in the pre -emptively treated. This was especially true in those fistula with a higher baseline Qa (P=0.001), showing that early intervention before Qa flow fell too low increased longevity.
Analysis into the cost effectiveness of vascular access surveillance and pre -emptive intervention is scant, with opposing sides arguing that the increased number of interventions may outweigh the overall reduction in access thrombosis61,62. It is known through experience that fistulas are less expensive to create and maintain and that, if done correctly, economic advantage lies not only in surveillance of these types of accesses, but also in increasing the number of patients with this particular type of vascular access.
SECONDARY ARTERIOVENOUS FISTULA
Although less favourable, AVG can be the initial or subsequent vascular access created for haemodialysis patients, for myriad reasons. Though evidence also recognises that due to the poor vascular health of a majority of ESRD patients in some cases placement of an AVG may be more beneficial due to low AVF maturation rates and the requirement of catheter insertion for temporary dialysis purposes63.
The US-based AV Fistula First Breakthrough Initiative is centred on 11 Change Concepts with Change Concept #6 suggesting Secondary AVF placement in patients with AVG, supported by the NFK–KDOQI Practice Guidelines11,64. Pre-existing forearm grafts lead to the arterialisation and dilatation of outflow veins in the ipsilateral arm and would facilitate creation of secondary AVF with often more than one anatomic site65,66. Several studies have looked at this approach with clear evidence of the success in conversion to secondary AVF, with a reduction of catheter use67-69.
Slayden reviewed two groups from different centres comparing the outcomes in a centre (Group 1) with a formal protocol in place for the pre-emptive assessment of conversion to secondary AVF with vein mapping, to one without such a protocol (Group 2)67.
In Group 1 92.5% underwent secondary AVF surgery prior to loss of AVG with a cumulative patency of 92.5% at year one and 87.5% at year two. In Group 2, only 19.3% were referred for secondary AVF surgery prior to loss of AVG with a cumulative patency of 94.4% at 1 year and 91.6% at 2 years. Cumulative patency rates exceeded 90% at 12 months in both groups, but dramatically fewer catheters were used in the centre with an existing conversion plan.
SUMMARY
The AVF remains the vascular access of choice in haemodialysis patients, but often requires initial investment of resources until the fistula is matured.
The physical examination, in trained hands, is both highly sensitive and specific at identifying stenosis and remains the cheapest option.
Therefore, more needs to be invested in developing these skills in both nephrology trainees and dialysis nursing staff. Pressure measurements have fallen out of favour due to the nature of the AVF circuit and have been largely superseded by blood flow analyses and recirculation as an indirect marker of stenosis. Direct visualisation with ultrasonography, magnetic resonance imaging and angiography (thought to be the gold standard), are both time consuming and require a large amount of resources and should not be used in routine surveillance.
Identifying a physiologically significant stenosiscan be challenging and may have negatively impacted outcomes in studies looking at percentage value of stenosis instead of an absolute diameter. Variations in interventional skills and techniques at different institutions may affect the documented long-term outcomes of stenosed fistulas, which are often complicated by restenosis secondary to neointimal hyperplasia.
More aggressive endovascular techniques with endoluminal stent insertion and drug-eluting technologies may hold the answer to guaranteeing long term access patency, in turn improving results seen in pre-emptive treatment of stenosis.
Endpoints in surveillance studies focus on fistula thrombosis, which occurs relatively seldom in AVF and more appropriate endpoints need to be considered.
Dialysis adequacy though a better reflection of fistula function remains difficult to quantify and grade and may require a scoring systems that is universal, making research studies easier and internationally translatable.
The growing number of patients with extensive vascular disease (e.g. diabetics) commencing haemodialysis has made the management of the AVF even more challenging. Therefore AVF surveillance needs to be done in a coordinated manner often requiring a dedicated staff member e.g. vascular access coordinator.
A vascular access coordinator may be involved in assisting the transition of patients from ESRD to haemodialysis, coordinating the long-term maintenance of the fistula, educating and training staff as well as coordinating data collection.
It is difficult to make recommendations for the ideal surveillance protocol as local experience and economics play a large part in what is possible in each healthcare system. The NFK-KDOQI Clinical Practice Guidelines prefer fistula blood flow measurements (Qa), physical findings and Doppler ultrasound11.
Flow measurements of less than 500ml/min and a trend in these findings over a period of time should prompt referral for more invasive testing.
Caring for Australasians with Renal Impairment (CARI) Guidelines suggest regular fistula blood-flow measurements (Qa) compared to physical examination, low arterial pressure and recirculation alone, as well as pre -emptive repair to reduce thrombosis rates and improve survival times70.
The latest available literature would suggest that three -monthly measurements of fistula blood flow (Qa), via ultrasound dilution technique, be completed with threshold values of Qa < 500ml/min in forearm fistulas and values higher for upper arm fistulas of possibly <750ml/min or > 25% reduction in Qa, be an indicator for more imaging e.g.formal angiograpghy or Doppler ultrasound. In combination with Qa, access recirculation of >5% with a non-urea based methods e.g. ultrasound dilution technique or >10% with a urea -based method, should also initiate further investigations. Pressure surveillance and traditional dialysis adequacy markers (Kt/V & URR) should not be used for fistula surveillance, given their inaccuracies and significant variability.
CONCLUSION
The dialysis population traditionally consumes a large amount of resources, due both to their complexity and multiple overlapping priorities and comorbidities.
The ongoing management of AVFs remains a challenge, with a growing urgency to improve outcomes for this expensive treatment modality, often restricted by the finite availability of resources in healthcare systems, most already unable to cope with demands.
It is thus understandable that some resistance exists to implement formalised surveillance programmes with the associated costs involved in staffing, purchasing of equipment and the resources required for endovascular and/or surgical interventions.
On the other hand, a programme is only as good as the cumulative experience and knowledge that has developed over time, and programmes that succeed involve a coordinated effort between multiple disciplines, which are essential in managing these patients.
A pre-emptive approach in creating systems to facilitate the transition of patients onto haemodialysis and maintaining them on this treatment with regular surveillance needs to be considered to minimise the future costs. The evidence above suggests that monitoring with additional support of more complex surveillance studies e.g. fistula blood flow, may allow AVF to be appropriately triaged for more invasive investigations and thus avoid inadequate dialysis and on rare occasions, fistula thrombosis.
References
1.Caskey F, Dawnay A, Farrington K, et al. 13th Annual Report of the Renal Association. Bristol, UK: UK Renal Registry; 2011 [ Links ]
2.Clayton PA, McDonald SP, Hurst K. 34th Annual ANZDATA Registry Report 2011 Summary. Adelaide, South Australia; 2011 [ Links ]
3.ERA-EDTA Annual Report 2010. Amsterdam, The Netherlands: Academic Medical Center, Department of Medical Informatics; 2012 [ Links ]
4.USRDS 2009 Annual Data Report: Atlas of End -Stage Renal Disease in the United States. Bethesda, MD: National Institues of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2009 [ Links ]
5.Oliver MJ, Rothwell DM, Fung K, Hux JE, Lok CE. Late creation of vascular access for hemodialysis and increased risk of sepsis. J Am Soc Nephrol 2004;5:936 -42 [ Links ]
6.Polkinghorne KR. Vascular access practice in hemodialysis: instrumental in determining patient mortality. Am J Kidney Dis 2009;53:359-62 [ Links ]
7.Polkinghorne KR, McDonald SP, Atkins RC, Kerr PG. Vascular access and all–cause mortality: a propensity score analysis. J Am Soc Nephrol 2004;5:477-86 [ Links ]
8.Feldman HI, Held PJ, Hutchinson JT. Hemodialysis vascular access morbidity in the United States. Kidney Int 1993;43:091-6 [ Links ]
9. Eggers P, Milam R. Trends in vascular access procedures and expenditures in Medicares ESRD program. In: Vascular Access for Hemodialysis. Chicago: WL Gore & Precept Press; 2001:133 -43 [ Links ]
10.Sehgal AR, Snow RJ, Singer ME. Barriers to adequate delivery of hemodialysis. Am J Kidney Dis 1998;31:593 -601 [ Links ]
11.NKF-KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for 2006 Updates: Hemodialysis Adequacy, Peritoneal Dialysis Adequacy and Vascular Access. Am J Kidney Dis 2006 (suppl 1);48:S1 -S322 [ Links ]
12.Sands JJ. Increasing AV fistulae and decreasing dialysis catheters: two aspects of improving patient outcomes. Blood Purif 2007;25:99 -102 [ Links ]
13.Lee H, Manns B, Taub K, et al. Cost analysis of ongoing care of patients with end–stage renal disease: the impact of dialysis modality and dialysis access. Am J Kidney Dis 2002;40:611 -22 [ Links ]
14.Allon M, Robbin ML. Increasing arteriovenous fistulas in hemodialysis patients: problems and solutions. Kidney Int 2002;62:1109 -24 [ Links ]
15.Turmel-Rodrigues L, Pengloan J, Rodrigue H, et al. Treatment of failed native arteriovenous fistulae for hemodialysis by interventional radiology. Kidney Int 2000;57:1124-40 [ Links ]
16.Paulson WD, Moist L, Lok CE. Vascular access surveillance: an ongoing controversy. Kidney Int 2012;81:132-42 [ Links ]
17.Lee T, Mokrzycki M, Moist L, Maya I, Vazquez M, Lok CE. Standardized definitions for hemodialysis vascular access. Semin Dial 2011;24:515-24 [ Links ]
18.Basile C, Ruggieri G, Vernaglione L, Montanaro A, Giordano R. The natural history of autogenous radio-cephalic wrist arteriovenous fistulas of haemodialysis patients: a prospective observational study. Nephrol Dial Transplant 2004;19:1231-6 [ Links ]
19.Allon M, Bailey R, Ballard R, et al. A multidisciplinary approach to hemodialysis access: prospective evaluation. Kidney Int 1998;53:473-9 [ Links ]
20.Dwyer A, Shelton P, Brier M, Aronoff G. A vascular access coordinator improves the prevalent fistula rate. Semin Dial 2012;25:239-43 [ Links ]
21.McQuillan R, Trpeski L, Fenton S, Lok CE. Modifiable risk factors for early mortality on hemodialysis. Int J Nephrol 2012;2012:435736 [ Links ]
22.Dinwiddie LC. Investing in the lifeline: the value of a vascular access coordinator. Nephrol News Issues 2003;17:49, 52 -3 [ Links ]
23.Polkinghorne KR, Seneviratne M, Kerr PG. Effect of a vascular access nurse coordinator to reduce central venous catheter use in incident hemodialysis patients: a quality improvement report. Am J Kidney Dis 2009;53:99-106 [ Links ]
24.Konner K, Hulbert -Shearon TE, Roys EC, Port FK. Tailoring the initial vascular access for dialysis patients. Kidney Int 2002;62:329-38 [ Links ]
25.Tessitore N, Lipari G, Poli A, et al. Can blood flow surveillance and pre -emptive repair of subclinical stenosis prolong the useful life of arteriovenous fistulae? A randomized controlled study. Nephrol Dial Transplant 2004;19:2325 -33 [ Links ]
26.Fahrtash F, Kairaitis L, Gruenewald S, et al. Defining a significant stenosis in an autologous radio-cephalic arteriovenous fistula for hemodialysis. Semin Dial 2011;24:231-8 [ Links ]
27.Tonelli M, James M, Wiebe N, Jindal K, Hemmelgarn B. Ultrasound monitoring to detect access stenosis in hemodialysis patients: a systematic review. Am J Kidney Dis 2008;51:630-40 [ Links ]
28.Beathard GA. Physical examination of the dialysis vascular access. Semin Dial 1998;11:231-6 [ Links ]
29.Beathard GA. The Physical Examination: The Forgotten Tool.: Lippincott Williams & Williams; 2002 [ Links ]
30.Sousa CN, Apostolo JL, Figueiredo MH, Martins MM, Dias VF. Physical examination: How to examine the arm with arteriovenous fistula. Hemodialysis international International Symposium on Home Hemodialysis 2012:235-41
31.Leon C, Asif A. Physical examination of arteriovenous fistulae by a renal fellow: does it compare favorably to an experienced interventionalist? Semin Dial 2008;21:557-60 [ Links ]
32.Maya ID, Oser R, Saddekni S, Barker J, Allon M. Vascular access stenosis: comparison of arteriovenous grafts and fistulas. Am J Kidney Dis 2004;44:859-65 [ Links ]
33.Campos RP, Chula DC, Perreto S, Riella MC, do Nascimento MM. Accuracy of physical examination and intra-access pressure in the detection of stenosis in hemodialysis arteriovenous fistula. Semin Dial 2008;21:269-73 [ Links ]
34.Asif A, Leon C, Orozco -Vargas LC, et al. Accuracy of physical examination in the detection of arteriovenous fistula stenosis. Clin J Am Soc Nephrol 2007;2:1191-4 [ Links ]
35.Mercado C, Salman L, Krishnamurthy G, et al. Early and late fistula failure. Clin Nephrol 2008;69:77-83 [ Links ]
36.Chawla A, DiRaimo R, Panetta TF. Balloon angioplasty to facilitate autogenous arteriovenous access maturation: a new paradigm for upgrading small-caliber veins, improved function, and surveillance. Semin Vasc Surg 2011;24:82-8 [ Links ]
37.De Marco Garcia LP, Davila -Santini LR, Feng Q, Calderin J, Krishnasastry KV, Panetta TF.Primary balloon angioplasty plus balloon angioplasty maturation to upgrade small-caliber veins (<3 mm) for arteriovenous fistulas. J Vasc Surg 2010;52:139-44 [ Links ]
38.Faiyaz R, Abreo K, Zaman F, Pervez A, Zibari G, Work J. Salvage of poorly developed arteriovenous fistulae with percutaneous ligation of accessory veins. Am J Kidney Dis 2002;39:824-7 [ Links ]
39.Miller GA, Goel N, Khariton A, et al. Aggressive approach to salvage non-maturing arteriovenous fistulae: a retrospective study with follow-up. J Vasc Access 2009;10:183-91 [ Links ]
40.Planken RN, Duijm LE, Kessels AG, et al. Accessory veins and radial-cephalic arteriovenous fistula non -maturation: a prospective analysis using contrast-enhanced magnetic resonance angiography. J Vasc Access 2007;8:281-6 [ Links ]
41.Nguyen VD, Robinson K, Griffith C. A guideline misguided: a review of the 2006 KDOQI Guideline on surveillance/monitoring and prophylactic angioplasty of hemodialysis grafts. J Vasc Access 2008;9:111-2 [ Links ]
42.Krivitski NM, MacGibbon D, Gleed RD, Dobson A. Accuracy of dilution techniques for access flow measurement during hemodialysis. Am J Kidney Dis 1998;31:502-8 [ Links ]
43.Magnasco A. Glucose pump test: a new method for blood flow measurement. Nephrol Dial Transplant 2002;17:2244-8 [ Links ]
44.Mercadal L, Hamani A, Bene B, Petitclerc T. Determination of access blood flow from ionic dialysance: theory and validation. Kidney Int 1999;56:1560-5 [ Links ]
45.Lindsay RM, Blake PG, Malek P, Posen G, Martin B, Bradfield E. Hemodialysis access blood flow rates can be measured by a differential conductivity technique and are predictive of access clotting. Am J Kidney Dis 1997;30:475-82 [ Links ]
46.Ronco C, Brendolan A, Crepaldi C, DIntini V, Sergeyeva O, Levin NW. Noninvasive transcutaneous access flow measurement before and after hemodialysis: impact of hematocrit and blood pressure. Blood Purif 2002;20:376-9 [ Links ]
47.Polkinghorne KR, Lau KK, Saunder A, Atkins RC, Kerr PG. Does monthly native arteriovenous fistula blood -flow surveillance detect significant stenosis–a randomized controlled trial. Nephrol Dial Transplant 2006;21:2498-506 [ Links ]
48.Sands JJ, Jabyac PA, Miranda CL, Kapsick BJ. Intervention based on monthly monitoring decreases hemodialysis access thrombosis. ASAIO J 1999;45:147-50 [ Links ]
49.Schwarz C, Mitterbauer C, Boczula M, et al. Flow monitoring: performance characteristicsof ultrasound dilution versus color Doppler ultrasound compared with fistulography. Am J Kidney Dis 2003;42:539-45 [ Links ]
50.Kleinekofort W, Kraemer M, Rode C, Wizemann V. Extracorporeal pressure monitoring and the detection of vascular access stenosis. Int J Artif Organs 2002;25:45-50 [ Links ]
51.Kleinekofort W. Improved measurement of vascular access pressure. J Vasc Access 2002;3:58-63 [ Links ]
52.Besarab A, Asif A, Roy-Chaudhury P, Spergel LM, Ravani P. The native arteriovenous fistula in 2007. Surveillance and monitoring. J Nephrol 2007;20:656-67 [ Links ]
53.Allon M, Robbin ML. Hemodialysis vascular access monitoring: current concepts. Hemodialysis international International Symposium on Home Hemodialysis 2009;13:153-62
54.Besarab A, Sullivan KL, Ross RP, Moritz MJ. Utility of intra -access pressure monitoring in detecting and correcting venous outlet stenoses prior to thrombosis. Kidney Int 1995;47:1364-73 [ Links ]
55.Magnasco A, Alloatti S. Glucose infusion test (GIT) compared with the saline dilution technology in recirculation measurements. Nephrol Dial Transplant 2006;21:3180-4 [ Links ]
56.Alloatti S, Magnasco A, Manes M, Bonfant G. [Hemodialysis access recirculation.]. G Ital Nefrol 2004;21:438-45 [ Links ]
57.Tessitore N, Bedogna V, Gammaro L, et al. Diagnostic accuracy of ultrasound dilution access blood flow measurement in detecting stenosis and predicting thrombosis in native forearm arteriovenous fistulae for hemodialysis. Am J Kidney Dis 2003;42:331-41 [ Links ]
58.Tessitore N, Mansueto G, Bedogna V, et al. A prospective controlled trial on effect of percutaneous transluminal angioplasty on functioning arteriovenous fistulae survival. J Am Soc Nephrol 2003;14:1623-7 [ Links ]
59.Tapping CR, Mallinson PI, Scott PM, Robinson GJ, Lakshminarayan R, Ettles DF. Clinical outcomes following endovascular treatment of the malfunctioning autologous dialysis fistula. J Med Imaging Radiat Oncol 2010;54:534-40 [ Links ]
60.Sugimoto K, Higashino T, Kuwata Y, Imanaka K, Hirota S, Sugimura K. Percutaneous transluminal angioplasty of malfunctioning Brescia-Cimino arteriovenous fistula: analysis of factors adversely affecting long-term patency. Eur Radiol 2003;13:1615-9 [ Links ]
61.Bittl JA, Cohen DJ, Seek MM, Feldman RL. Economic analysis of angiography and preemptive angioplasty to prevent hemodialysis -access thrombosis. Catheter Cardiovasc Interv 2010;75:14-21 [ Links ]
62.Tessitore N, Bedogna V, Poli A, et al. Adding access blood flow surveillance to clinical monitoring reduces thrombosis rates and costs, and improves fistula patency in theshort term: a controlled cohort study. Nephrol Dial Transplant 2008;23:3578-84 [ Links ]
63.Rosas SE, Feldman HI. Synthetic vascular hemodialysis access versus native arteriovenous fistula: a cost-utility analysis. Ann Surg 2012;255:181-6 [ Links ]
64.Change Concepts. (Accessed 10.7.2011, 2011, at http://fistulafirst.org/Professionals/FFBIChangeConcepts.aspx. [ Links ])
65.Beathard GA. Interventionalists role in identifying candidates for secondary fistulas. Semin Dial 2004;17:233-6 [ Links ]
66.Nguyen VD, Treat L, Griffith C, Robinson K. Creation of secondary AV fistulas from failed hemodialysis grafts: the role of routine vein mapping. J Vasc Access 2007;8:91-6 [ Links ]
67.Slayden GC, Spergel L, Jennings WC. Secondary arteriovenous fistulas: converting prosthetic AV grafts to autogenous dialysis access. Semin Dial 2008;21:474-82 [ Links ]
68.Asif A, Unger SW, Briones P, et al. Creation of secondary arteriovenous fistulas: maximizing fistulas in prevalent hemodialysis patients. Semin Dial 2005;18:420-4 [ Links ]
69.Salman L, Alex M, Unger SW, Contreras G, Lenz O, Asif A. Secondary autogenous arteriovenous fistulas in the fistula first era: results of a longterm prospective study. J Am Coll Surg 2009;209:100-5 [ Links ]
70.Polkinghorne KR. Vascular access surveillance. Nephrology 2008;13:S1-S11 [ Links ]
Dr Martin A. Feddersen
Department of Renal Medicine, Gosford Hospital
Gosford 2250, NSW, Australia
E-mail: martinfeddersen@yahoo.com.au
Conflict of interest statement. None declared.
Acknowledgements: Jan Swinnen & Lukas Kairaitis for providing invaluable advice and support in understanding the physiology of arteriovenous fistulas and providing figures outlining fistula anatomy.
Received for publication: 26/10/2012
Accepted: 06/11/2012














