Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.26 no.2 Lisboa abr. 2012
Pericardial and pleural effusions associated with sirolimus and discussion of possible mechanisms
Sofia Rocha, Sofia Pedroso, Manuela Almeida, Leonídio Dias, La Salete Martins, António C. Henriques, António Cabrita
Department of Nephrology and Transplantation, Hospital Geral de Santo António.
Oporto, Portugal.
ABSTRACT
Sirolimus, a mammalian target of rapamycin inhibitor, is an increasingly used immunosuppressant in solid-organ transplantation. There are an increasing number of reports of unusual oedematous adverse effects associated with this drug, including lymphoedema, ascites and pleural effusions, and a few reports of pericardial effusions.
No pathophysiological explanation for these phenomena has been disclosed.
We report a 33-year-old sirolimus-treated kidney transplant recipient with chronic pericardial and pleural effusions identified nine years after transplantation.
He was initially treated for a presumed tuberculous pericarditis, even though cultures for Mycobacterium tuberculosis were negative. After 12 months of antitubercular therapy, visceral effusions persisted. Pericardial effusion was drained and stabilised.
After exclusion of other causes, sirolimus toxicity was considered the most likely cause. Two months after discontinuation of sirolimus, visceral effusions disappeared.
Interaction of mammalian target of rapamycin inhibitors with mediators of lymphangiogenesis may be a common link in oedematous states associated with sirolimus.
Key-Words: Immunosuppression; kidney transplantation; lymphangiogenesis; mTOR inhibitors; rapamycin; serositis.
INTRODUCTION
Sirolimus is a macrolide antibiotic used for prophylaxis of rejection in patients who have received solid-organ transplants.
Common side effects of sirolimus include dyslipidaemia, proteinuria, anaemia, thrombocytopenia, decreased wound healing, oral ulcers and lymphoceles. There have been an increasing number of reports of other rarer complications of sirolimus, including interstitial pneumonitis, pulmonary alveolar proteinosis1, lymphoedaema2-7, chylous ascites8, and pericardial effusions6,9-11. The mechanisms of these adverse reactions have not been fully elucidated. In 2008, a pharmacovigilance review11 suggested a possible association between sirolimus and the occurrence of pericardial effusion, most notably in cardiac transplant recipients.
In kidney transplant recipients we are aware of two reports of pericardial effusion associated with sirolimus6,10.
We report a kidney transplant recipient for nine years with chronic pleural and pericardial effusions, which we believe were attributed to sirolimus. The anti-lymphangiogenic activity of mTOR inhibition may be a common denominator in the pathophysiology of oedematous states associated with sirolimus.
CASE REPORT
A 33-year-old male with a status of post-deceased donor kidney transplant 12 years prior was admitted to our department with fever and chronic pericardial and pleural effusions.
In 1994, at the age of 17 years, hypertension and advanced chronic kidney disease was diagnosed, presumed to be due to chronic glomerulonephritis. Two years later he started haemodialysis.
In 1998, he received a kidney transplant from a deceased-donor. Induction of immunosuppression consisted of sirolimus, ciclosporin and steroids as part of a study protocol12. He had immediate graft function and was discharged with a serum creatinine of 1.8 mg/dL. His wound healing was unremarkable, and kidney function remained stable with a serum creatinine around 1.5 mg/dL.
In 2005, his serum creatinine increased to 2.5 mg/dL and he developed proteinuria (1 g/day). Ciclosporin was suspended and he maintained sirolimus (trough levels 6-8 ng/mL) and prednisolone. Renal allograft function slightly improved and remained stable with serum creatinine around 2.0 mg/dL and proteinuria 1 g/day. In 2006, a chest X-ray shown an increased cardiac silhouette, attributed to left ventricular hypertrophy.
In 2007, he was admitted to our department with fever and dry cough lasting for a week. His temperature was 38.2ºC and blood pressure 141/91 mmHg. Arterial blood gases (FiO2: 21%) pO2: 65 mmHg. Laboratory findings revealed a CRP of 200 mg/L, a serum creatinine of 2.4 mg/dL and a sirolimus level of 6.3 ng/mL. Chest X-ray showed bilateral pleural effusions, more pronounced on the right. He started levofloxacin and cefotaxime to treat a community-acquired pneumonia. After 72 hours of therapy, fever persisted, and a CT scan was performed to rule out infectious complications, namely empyema and lung abscess. A right inferior lobe lung condensation, small bilateral pleural effusions and a moderate-large pericardial effusion were identified (Fig. 1); there were no signs of interstitial pneumonitis. Transthoracic echocardiogram did not show pericardial tamponade or ventricular systolic dysfunction. Levofloxacin and cefotaxime were maintained until the patient underwent bronchoscopy, which ruled out Pneumocystis jiroveci, other opportunistic infections and lymphocytic alveolitis. Mycobacterium tuberculosis culture of bronchoalveolar lavage was negative. Serologic studies for virus and parasites were negative. Because there were no signs of pericardial tamponade and the diagnostic yield was low, we did not undertake pericardiocentesis. A presumptive diagnosis of tuberculous pericarditis and pleuritis was made, and anti-tubercular therapy was started with three drugs: isoniazid, rifampicin and pyrazinamide. Fever disappeared after five days of added anti-tubercular therapy, and therapy was maintained for 12 months. His immunological study and thyroid function were normal. Visceral effusions persisted and were considered most likely idiopathic. The patient did not report chest pain and had no other signs of fluid overload. His serum creatinine remained stable at around 2.0-2.5 mg/dL.
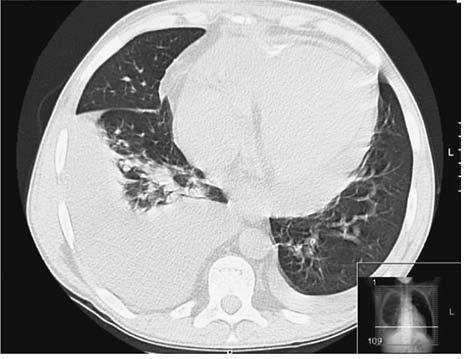
Figure 1
Thoracic CT scan showing bilateral pleural effusions more pronounced on the right side, with lower inferior lobe lung consolidation, and a moderatelarge volume pericardial effusion.
In 2009, even though there were no signs of haemodynamic disturbances aggravating clinical evolution, an elective pericardiocentesis was undertaken, due to the risk of sudden cardiac tamponade. The procedure was complicated by right ventricle laceration and cardiac tamponade. He underwent surgical drainage of the pericardial effusion and pericardial window and biopsy. Pericardial fluid was a sterile exudate without malignant cells; adenosine deaminase (ADA) was 17 U/L (N< 22 U/L); PCR for Mycobacterium tuberculosis was negative. Pericardial biopsy revealed pericardial fibrosis and mesothelial hyperplasia, without malignant cells. The patient was discharged with a serum creatinine of 3.3 mg/dL. Further echocardiogram showed a small pericardial effusion.
Two months later, he was readmitted with fever and aggravated pleural effusions. He referred fever and dry cough lasting for three days. Laboratory data revealed CRP 280 mg/L, serum creatinine 3.9 mg/dL, and sirolimus 6.7 ng/mL. CT scan showed a volume increase of pleural effusions, with no abnormal lung findings. Echocardiogram findings showed a small volume pericardial effusion, with no signs of cardiac tamponade. Thoracocentesis revealed a sterile exudates with negative ADA. Due to presumed communityacquired respiratory infection, we started levofloxacin, and fever disappeared 48 hours later. Without any other obvious aetiology for the chronic effusions, sirolimus was considered the most likely cause, and was replaced by mofetil mycophenolate.
Two months after sirolimus discontinuation, pleural and pericardial effusions had completely regressed.
The patient did not receive any other specific treatment, including diuretics. At 10 months of follow-up, repeat chest X-ray was clear and echocardiogram showed no pericardial effusion. His kidney allograft function improved (serum creatinine 2.7 mg/dL), as did proteinuria (1.2 g/day) and anaemia (Hb: 13 g/dL, without erythropoiesis stimulating agents).
DISCUSSION
We report a kidney transplant recipient under sirolimus with chronic pericardial and pleural effusions.
We considered sirolimus the most likely cause of these phenomena.
The diagnosis of drug-related toxicity is usually suggested by the presence of the following criteria: (i) exposition to the drug precedes the occurrence of signs and/or symptoms (ii) there is no alternative cause for the associated syndrome and (iii) withdrawal of the drug leads to resolution of the signs and/or symptoms.
Nine years after the kidney transplant, our patient presented a febrile episode associated with respiratory complaints; a lung condensation that failed to respond to empiric therapy with levofloxacin. This led us to undertake a CT scan to rule out infectious complications. A moderate-large pericardial effusion and bilateral pleural effusions were disclosed. Due to the size of the pericardial effusion and the absence of cardiac tamponade, we presumed that the effusion was slowly progressive. Chronic effusive pericarditis has a broad range of differential diagnosis including infectious, immunological, neoplastic, endocrine, idiopathic and drug-related causes. In this case, there was no previous history of viral infection or acute pericarditis, and serologic tests were negative. Hypothyroidism, neoplasm and immunological diseases were ruled out, and uraemia seemed a very unlikely cause, as the patients creatinine clearance was over 30 ml/min. Tuberculosis seemed the most likely diagnosis, even though the cultures for Mycobacterium tuberculosis were negative. The incidence of tuberculosis in Portugal is quite high13, and solid-organ transplant recipients have a marked increase in its frequency and severity compared to the general population. Persistence of visceral effusions after adequate treatment of tuberculosis led us to consider sirolimus as a potential causal agent. After reviewing the literature, we found an increasing number of reports of adverse oedematous events attributed to sirolimus.
Lymphoceles are well-described adverse events of sirolimus, with an incidence of up to 38%14. They result from lesions to the lymphatic network by surgical procedures, trauma, infection or chronic inflammation. In addition to lymphoceles, cases of uni- or bilateral eyelid oedema15 and lymphoedema2-7 have also been reported. We are aware of 19 reports of lymphoedema associated with sirolimus, either localised2-5,7 or generalised6. De Bartolomeis et al.6 reported a kidney transplant recipient who developed incapacitating generalised lymphoedema, ascites, and pleural and pericardial effusions. Three months after sirolimus discontinuation, lymphoedema and visceral effusions resolved completely. Our group has described a kidney transplant recipient who developed chylous ascites unrelated to surgical injury one year after conversion to sirolimus8. After exclusion of other causes, sirolimus was considered the most likely aetiology of ascites. One month after sirolimus withdrawal, ascites disappeared and did not recur.
In 2004, Montalbano et al.9 described four cases of pericardial effusion in sirolimus-treated liver transplant recipients. Three of these patients also had pleural effusions. A workup of infectious causes was undertaken and was negative. All the patients had resolution of visceral effusions after sirolimus discontinuation.
In 2005, Truong et al.10 described for the first time the occurrence of a pericardial effusion requiring pericardiocentesis in a sirolimus-treated paediatric kidney transplant recipient. Although the patient had intercurrent adenoviral infection, sirolimus was considered the most likely cause of pericardialeffusion. After sirolimus dose reduction, pericardial effusion stabilised and did not recur. In 2008, a pharmacovigilance review11 identified 56 cases (35 in kidney transplant recipients) of pericardial effusion coincident with sirolimus therapy, regardless of coexistence of other possible causes (i.e. cardiac trauma, and viral or drug-induced pericarditis). In cardiac transplant recipients under sirolimus, the risk of pericardial effusion was substantially higher when compared to patients under azathioprin (28% vs. 9%, P=0.014). In kidney transplant recipients, the incidence of pericardial effusion was 0.7-2.3%, with a median time to onset of 38 weeks (range 3-202 weeks) from grafting. They found no differences in the incidence of pericardial effusion between patients treated de novo or patients converted to sirolimus, nor did they mention any correlation of this adverse effect to sirolimus blood concentration. The incidence of cardiac tamponade was 6.6%, and approximately half (31 of 56) of the patients required pericardial drainage. They concluded that the available literature suggests a possible association between sirolimuscontaining immunosuppressant regimens and the occurrence of pericardial effusion, most remarkably in cardiac transplant recipients.
Sirolimus-related oedematous adverse events may share a common link. The lymphatic network is responsible for the interstitial fluid balance and clearance of macromolecules and represents an important route for circulation of immune cells, as well as for metastatic tumour cells. Under pathological conditions such as trauma, surgical disruption, infection, chronic inflammation and cancer, there is lymphatic vessel proliferation through stimulation of various growth factors, including vascular endothelial growth factor (VEGF) C and D, acting via VEGF receptor 3 (VEGFR-3).
Mutated variants of this receptor are responsible for congenital lymphoedemas. In 2007, Hubert et al.16 investigated whether mTOR inhibition, in addition to its antihemangiogenic effect, also impedes regenerative lymphangiogenesis. In a murin skin flap model, sirolimus impaired the growth of lymphatic endothelial cells and lymphatic regenerations in surgical incisions, resulting in prolonged macroscopic oedema. Even concentrations of sirolimus as low as 1 ng/mL inhibited VEGF-C driven proliferation and migration of lymphatic endothelial cells. The inhibitory effect of sirolimus on VEGF-C expression was also shown in a metastasis animal model, in which the number and area of lymphatic vessels in primary tumors were significantly decreased by this drug17.
These data provide a possible explanation for some of the reported adverse effects of sirolimus, including impaired wound healing, lymphoceles, lymphoedema and visceral effusions. They are also a rationale for the use of this drug in certain pathological conditions, such as some types of cancer and lymphangiomiomatosis, a rare systemic disease in which there is over-expression of VEGF-C18. Despite the increasing number of reports of oedematous effects (peripheral and visceral) associated with sirolimus, their incidence is very low, suggesting multifactorial mechanisms. Lymphatic insufficiency induced by surgical trauma, infection and inflammation can partly explain the occurrence of these phenomena. It is possible that genetic susceptibility and a pre-existing constitutive lymphatic insufficiency contribute to the development of these complications in sirolimus-treated patients.
In this case, the mechanisms by which visceral effusions occurred remain obscure, as we could not identify any preceding traumatic, infectious or inflammatory event. We also do not know exactly how long after the introduction of sirolimus the visceral effusions occurred, since they were incidental findings nine years after grafting. In other reports, visceral effusions occurred earlier after introduction of sirolimus (maximum five years11). We cannot say with absolute certainty whether our patients visceral effusions were causally related to sirolimus. However, we cannot find alternative explanations for these phenomena, and there was clearly a time relationship between suspension of sirolimus and their complete resolution.
We conclude that, after exclusion of other causes, sirolimus should be considered a potential cause of visceral effusions. Inhibition of lymphangiogenesis is a putative mechanism for this association.
References
1. Pedroso SL, Martins LS, Sousa S, et al. Pulmonary alveolar proteinosis: a rare pulmonary toxicity of sirolimus. Transpl Int 2007;20:291-6 [ Links ]
2. Aboujaoude W, Milgrom ML, Govani MV. Lymphedema associated with sirolimus in renal transplant recipients. Transplantation 2004;77:1094-6 [ Links ]
3. Romagnoli J, Citterio F, Nanni G, Tondolo V, Castagneto M. Severe limb lymphedema in sirolimus-treated patients. Transplant Proc 2005;37:834-6 [ Links ]
4. Al-Otaibi T, Ahamed N, Nampoory MR, et al. Lymphedema: an unusual complication of sirolimus therapy. Transplant Proc 2007;39:1207-10 [ Links ]
5. van Onna M, Geerts A, Van Vlierberghe H, et al. One-sided limb lymphedema in a liver transplant recipient receiving sirolimus. Acta Gastroenterol Belg 2007;70:357-9 [ Links ]
6. De Bartolomeis C, Collini A, Rumberger B, et al. Generalized lymphedema in a sirolimustreated renal transplant patient. Clin Transplant 2008;22:254-7 [ Links ]
7. Desai N, Heenan S, Mortimer PS. Sirolimus-associated lymphoedema: eight new cases and a proposed mechanism. Br J Dermatol 2009;160:1322-6 [ Links ]
8. Castro G, Freitas C, Beirão I, Rocha G, Henriques AC, Cabrita A. Chylous ascites in a renal transplant recipient under sirolimus (rapamycin) treatment. Transplant Proc 2008;40:1756-8 [ Links ]
9. Montalbano M, Neff GW, Yamashiki N, et al. A retrospective review of liver transplant patients treated with sirolimus from a single center: an analysis of sirolimus-related complications. Transplantation 2004;78:264-8 [ Links ]
10. Truong U, Moon-Grady AJ, Butani L. Cardiac tamponade in a pediatric renal transplant recipient on sirolimus therapy. Pediatr Transplant 2005;9:541-4 [ Links ]
11. Steele GH, Adamkovic AB, Demopoulos LA, et al. Pericardial effusion coincident with sirolimus therapy: a review of Wyeths safety database. Transplantation 2008;85:645-7 [ Links ]
12. Ruiz JC, Campistol JM, Grinyó JM, et al. Early cyclosporine a withdrawal in kidneytransplant recipients receiving sirolimus prevents progression of chronic pathologic allograft lesions. Transplantation 2004;78:1312-8 [ Links ]
13. Couceiro L, Santana P, Nunes C. Pulmonary tuberculosis and risk factors in Portugal: a spatial analysis. Int J Tuberc Lung Dis 2011 Jul 6. [Epub ahead of print] [ Links ]
14. Langer RM, Kahan BD. Incidence, therapy, and consequences of lymphocele after sirolimus-cyclosporine-prednisone immunosuppression in renal transplant recipients. Transplantation 2002;74:804-8 [ Links ]
15. Mohaupt MG, Vogt B, Frey FJ. Sirolimus-associated eyelid edema in kidney transplant recipients. Transplantation 2001;72:162-4 [ Links ]
16. Huber S, Bruns CJ, Schmid G, et al. Inhibition of the mammalian target of rapamycin impedes lymphangiogenesis. Kidney Int 2007;71:771-7 [ Links ]
17. Kobayashi S, Kishimoto T, Kamata S, Otsuka M, Miyazaki M, Ishikura H. Rapamycin,a specific inhibitor of the mammalian target of rapamycin, suppresses lymphangiogenesis and lymphatic metastasis. Cancer Sci 2007;98:726-33 [ Links ]
18. Kumasaka T, Seyama K, Mitani K, et al. Lymphangiogenesis mediated shedding of LAM cell clusters as a mechanism for dissemination in lymphangioleiomyomatosis. Am J Surg Pathol 2005;291356-66 [ Links ]
Dr Sofia Rocha
Department of Nephrology
Hospital de Santo António
Largo Professor Abel Salazar
4099-001 Porto, Portugal
Email:sofiarocha81@gmail.com
Conflict of interest statement.None declared.
Received for publication: 05/12/2011
Accepted in revised form: 07/02/2012














