Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Nephrology & Hypertension
versão impressa ISSN 0872-0169
Port J Nephrol Hypert vol.26 no.2 Lisboa abr. 2012
An update on immunosuppression for the HIV-positive kidney transplant recipient
Ana Natário1, Patrícia Matias2, André Weigert2,3
1 Department of Nephrology, Centro Hospitalar de Setúbal. Setúbal, Portugal.
2Department of Nephrology, Hospital de Santa Cruz. Carnaxide, Portugal.
3Department of Pharmacology and Neurosciences, Lisbon Medical School. Lisbon, Portugal.
ABSTRACT
The outcome of human immunodeficiency virus (HIV)-positive patients has improved dramatically with the advent of combined antiretroviral therapy.
The mortality rate for HIV-positive patients with chronic kidney disease stage 5 is now similar to those without HIV infection, making kidney transplantation an increasingly considered alternative treatment for end-stage renal disease in this population.
Knowledge of the pharmacokinetics of antiretroviral medications and potential drug-drug interactions between antiretroviral and immunosuppressive medications are critical to the success of transplantation in this setting. The aim of this article is to present the state of the art kidney transplant therapy in HIV-positive patients.
Key-Words: Antiretroviral therapy; HIV; immunosuppression; kidney transplantation.
INTRODUCTION
Human immunodeficiency virus (HIV) infection is no longer an absolute contraindication for transplantation1.
Since the introduction of effective combined antiretroviral regimens which promote immunological recovery and suppression of viral replication, the outcome of HIV patients has improved dramatically.
Several studies demonstrate that the mortality rate for HIV-positive patients with end-stage renal disease (ESRD) is now similar to those without HIV infection2,3.
Given this significant improvement in HIV patients life expectancy, kidney transplantation has been increasingly considered as an alternative treatment for ESRD in this population4.
In recent years, more than 200 renal transplants have been performed in HIV-infected patients worldwide1.
According to the Portuguese Society of Nephrology the prevalence of HIV-positive patients on haemodialysis in Portugal in 2010 was close to 1.2% of all dialysis patients or, approximately, 120 patients5 . There are no specific registries of transplantation in HIV-positive patients in Portugal, but as of today, five kidney transplants have been performed in four centres nationwide, according to a personal inquiry to all transplant units.
All nephrologists are likely to take care of HIVpositive patients with kidney disease and therefore need to be acquainted with the pharmacokinetics of antiretroviral medications and proper dosing of these drugs at different stages of chronic kidney disease (CKD). Furthermore, awareness of drug interactions between antiretroviral and immunosuppressive medications is of paramount importance for the success of transplantation in these individuals. Close monitoring of drug levels is also critical due to the robust interactions that can be observed6.
KIDNEY TRANSPLANTATION IN THE PRE-CART ERA
Before the advent of combined antiretroviral therapy (cART), reports on kidney transplantation in HIVpositive patients were limited to either isolated case reports or to a small number of patients4 because of the potential risks of immunosuppression in the context of unleashed HIV infection7. One of the first reports, released in the early 90s, showed a 36% mortality rate and a graft survival of only 54.9% at 30 months4.
The largest review of kidney transplantation in HIV patients over the pre-cART period was obtained from the United States Renal Data System (USRDS) and analysed kidney transplantations performed between 1987 and 1997. The results showed both a worse five-year patient survival (71% versus 78%) and kidney graft survival (44% versus 61%) when HIV-positive recipients of kidney allografts were compared to seronegative individuals. In multivariate analysis, HIV infection was listed as an independent risk factor for mortality, as well as for graft loss, in kidney transplant recipients from deceased donors8.
KIDNEY TRANSPLANTATION IN THE POST-CART ERA
After the advent of cART in 1996, life expectancy in HIV-infected patients changed significantly, with a marked decrease in morbidity and mortality rates4.
In addition to being effective in treating established HIV-associated nephropathy (HIVAN), cART may also potentially decrease the actual incidence of novo HIVAN 9,10. However, after several years of cART therapy, some patients eventually progress to ESRD with the mechanisms remaining to be elucidated11.
It is important to keep in mind that several components of cART, with particular emphasis on the protease inhibitors, may impact on cardiovascular and metabolic risk factors which cause or accelerate kidney disease.
A recent analysis of the USRDS confirmed that the mortality in HIV patients receiving deceased kidneys in the cART era had improved dramatically, although black patients tended to be underrepresented12. Most transplant groups from Europe and North America considered the following criteria for including HIVinfected patients on the transplant waiting list: clinical (no AIDS-defining diseases), immunological (CD4T-cell count above 200 cells/μl), virological (undetectable HIV viral load in plasma, i.e. < 50 copies/ml for at least 6 months) and social (appropriate degree of stability with no active consumption of drugs or alcohol and adherence to proposed therapies)1.
Current therapeutic HIV guidelines recommend the initiation of cART in individuals presenting with less than 350 CD4 T-cells/μl or with concurrent morbidities, namely HIVAN13.
Contemporary treatment of HIV infection involves the combination of at least three fully active drugs from the currently available classes of antiretroviral medications: nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors (PIs), entry inhibitors and integrase inhibitors. The current antiretroviral regimens considered to naïve patients are based on a combination of two NRTIs and a boosted PI, an NNRTI or an integrase inhibitor13,14.
Some antiretroviral medications need to be adjusted to kidney function, namely NRTIs (Table I). For patients with CKD, dose adjustments are not necessary for NNRTIs (delavirdine, efavirenz, etravirine, nevirapine); PIs (atazanavir, darunavir, fosamprenavir, indinavir, lopinavir/ritonavir, nelfinavir, ritonavir, saquinavir, tipranavir); C-C chemokine receptor type 5 (CCR5) antagonists (maraviroc); fusion inhibitors (enfurvitide); or integrase inhibitors (raltegravir)13.
Table I
Antiretroviral dosing recommendations in HIV infected adults according to creatinine clearance (adapted from 11)
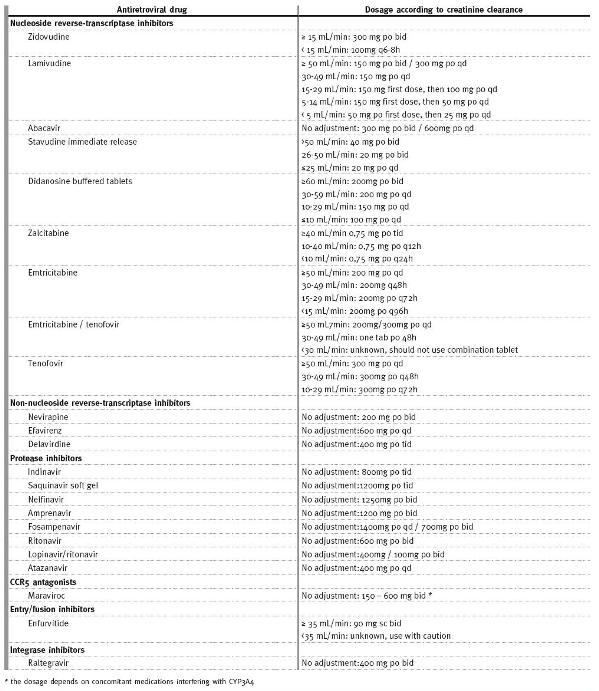
Despite the fact that tenofovir has been associated with nephrotoxicity, a large observational study in Africa failed to detect worsening of kidney function in patients treated with this NRTI. In this study, almost all cases of deterioration of kidney function were attributed to well-established causes other than this drug15. It is important to mention that tenofovir is particularly attractive in regions with high prevalence of hepatitis B, due to the dual effect against both viruses. Nevertheless, due to the potential to increase serum creatinine at least in some patients, alternative therapies such as abacavir may be preferable to treat kidney allograft recipients as other toxic, immunologic and haemodynamic factors may affect kidney function in this specific group of patients and tenofovirs effect may be a confounding factor.
I MMUNOSUPPRESSION AFTER KIDNEY TRANSPLANTATION IN HIV-POSITIVE PATIENTS
With the dramatic reductions in HIV-associated morbidity and mortality observed since the availability of cART, the safety of immunosuppression in this population has become the more pressing concern.
Surprisingly, immunosuppression may have a beneficial impact on patients with HIV infection by reducing the pool of activated T-cell targets for new infection, decreasing the immune activation characteristic of HIV pathogenesis, inhibiting HIV replication, and/or interacting synergistically with antiretroviral agents7.
A critical point for transplantation is the selection of the optimal immunosuppression protocol to prevent rejection, which requires the modulation of the immune system in a group of patients displaying an immunological dysfunction, and therefore more prone to opportunistic infections, but, on the other hand, with an intact and probably even enhanced potential for allorecognition16,17.
1 – Induction therapy
In the initial clinical trials of organ transplantation in HIV-positive patients, immunosuppressive regimens focused on maintenance therapy with agents with known antiretroviral qualities. This therapy consisted of a combination of steroids, a calcineurin inhibitor (CNI) and mycofenolate mofetil (MMF). However, organ recipients with HIV infection can mount an alloimmune response and HIV-positive renal transplant recipients have a higher rejection rate than their counterparts without HIV17. The reason for such high rejection rates is unclear, although dysregulation of the immune system or insufficient immunosuppression are two possible causes2.
For this reason, induction therapy with interleukin-2 receptor inhibitor (basiliximab) was successfully introduced2,18. Most transplant centres are reluctant to use lymphocyte depleting agents for induction (thymoglobulin, alemtuzumab) as these agents severely deplete CD4 T-cells for several months. Nevertheless, these potent agents have successfully reversed aggressive rejection in several HIV-positive kidney transplant recipients19.
2 – Maintenance therapy
In a recent meta-analysis of twelve published case series16, maintenance immunosuppression therapy in HIV-positive kidney transplant recipients consisted most commonly of triple therapy with a CNI (ciclosporin as the most frequently used), MMF and steroids.
Some studies have demonstrated that some of the immunosuppressive drugs used in transplantation, such as CNI, sirolimus and mycophenolic acid, exhibit an antiretroviral action4 (Table II).
Table II
Effects of immunosuppressive drugs on antiretroviral activity
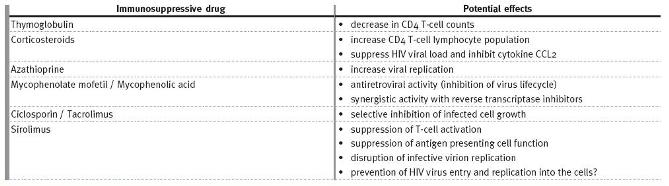
Corticosteroids- Izzedine et al. showed that prednisolone increases CD4 T-cell lymphocyte population20.
Another study showed that prednisolone acts by suppressing HIV viral load and inhibiting CCL2, a proinflammatory cytokine induced by HIV infection19.
Antimetabolic agents- Azathioprine use has been associated with increased viral replication, while the opposite seems to happen with the use of mycophenolic acid or its prodrug MMF20. Its virostatic action is thought to result from the depletion of guanoside nucleosides, which are necessary for the virus lifecycle and subsequent synergistic activity with NRTIs, namely abacavir, didanosine and tenofovir.
However, it negatively affects the action of zidovudine and stavudine21.
Calcineurin inhibitors- Ciclosporin and tacrolimus have well-documented antiretroviral effects through selective inhibition of infected cell growth. These agents interfere with HIV pathogenic protein functions, which ultimately results in the reduction of virus formation2. CNIs can however cause glucose intolerance, which can be exacerbated by concomitant administration of some antiretroviral agents.
Mamalian target of rapamycin (mTOR) inhibitors- Similarly to CNIs, sirolimus, a macrolid antibiotic produced by the fungus Streptomyces hygroscopicus, also exerts some antiretroviral activity through suppression of T-cell activation, suppression of professional antigen presenting cell function and disruption of infective virion replication. Sirolimus decreases the expression of CCR5 on monocytes and lymphocytes, thus potentially preventing the HIV virus from entering these cells and replicating22. This receptor may be a common link between HIV infection and allorecognition, as lower transplant rejection rates are observed in individuals expressing genetic deficiency (a 32 base pair deletion) at the CCR5 or following CCR5 blockade23. This concept is consistent
with the possibility that CCR5 antagonists such as maraviroc may have a role in prolonging graft survival in solid organ and bone marrow transplantation, raising also the possibility that sirolimus may be a reasonable option to treat the HIV-positive renal transplant recipient22.
3 – Rejection therapy
There are no current recommendations on how to treat rejection episodes in HIV-positive kidney transplant recipients24. The use of antilymphocyte polyclonal antibodies is controversial and many authors recommend restricting this therapy for patients with a very high immunological risk for rejection19,25. Thymoglobulin, an agent frequently used to manage acute rejection, may be associated with marked CD4 T-cell count depletion4. Several studies have reported significant decreases in CD4 T-cell counts in HIV-infected recipients related to the use of thymoglobulin3,25. In a study published by Stock et al. 3, the median change in the CD4 T-cell count from baseline to one year was greater in patients who received induction therapy with thymoglobulin compared to those who did not (-239 versus -135 cells per mm3). However, these changes were transient and the median change in CD4 T-cell count from baseline to 3 years was not significantly different between these groups (-57 and -52 cells per mm3, respectively). In addition, another study25 failed to detect an increased risk of opportunistic infections and progression to AIDS or death related to the use of lymphocyte depleting agents.
DRUG INTERACTIONS BETWEEN IMMUNOSUPPRESSIVE AGENTS AND CART
Pharmacokinetic interaction between antiretrovirals and immunosuppressants is the most intricate issue in organ transplantation of HIV-positive patients.
The administration of a complex immunosuppressive regimen in combination with antiretroviral therapy can result in an altered exposure to immunosuppressants and may be associated with rejection1.
In most centres, allograft recipients with HIV infection receive the same cART regimens they received before transplantation2. Early studies demonstrate that with this strategy, HIV-infected patients do not progress to AIDS17,18,26. Initial experience also suggests that these recipients can tolerate cART withdrawal for several weeks without changes in viral load and CD4 T-cell count26,27. Nevertheless, potential drug-drug interactions should be taken into consideration when selecting an antiretroviral regimen (Table III).
Table III
Drug interactions between antiretroviral agents and immunosuppressive drugs (adapted from 1)
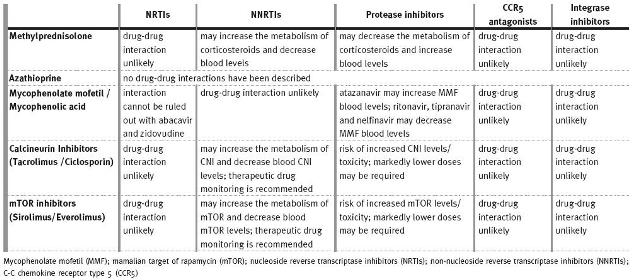
Most drug interactions with antiretroviral drugs are mediated through inhibition or induction of hepatic drug metabolism13. The most notable drug interaction occurs between antiretroviral medications and immunosuppressive agents that induce or inhibit the P-glycoprotein (P-gp) efflux transporters and CYP450 3A (CYP3A4) metabolising enzymes found in the gut and liver. These interactions can lead to unexpected increases or decreases in drug plasma levels, and result in toxic side effects, organ rejection or HIV disease breakthrough2.
All PIs and NNRTIs are metabolised in the liver by the CYP3A4 isoenzyme. Some examples of other drugs include medications that are commonly prescribed for non-HIV medical conditions, such as lipidlowering agents (e.g. statins), benzodiazepines, calcium channel blockers (e.g. diltiazem), immunosuppressants (e.g., CNIs and mTOR inhibitors), anticonvulsants, rifamycins, erectile dysfunction agents (e.g., sildenafil), ergot derivatives, azole antifungals, macrolides, oral contraceptives and methadone13.
The use of a CYP3A4 substrate that has a narrow margin of safety in the presence of a potent
CYP3A4 inhibitor may lead to markedly prolonged elimination half-life and toxic drug accumulation.
Avoidance of concomitant use or dose reduction of the affected drug, with close monitoring for doserelated toxicities, may be warranted13. Three frequently used groups of drugs are very robust blockers of CYP3A4: diltiazem, imidazolic antifungals (such as ketoconazol) and macrolides (erythromycin, clarithromycin). It is also relevant to mention that among antiretroviral drugs, ritonavir is specifically used as a booster, meaning that this less expensive PI is specifically used to block CYP 3A4 to allow lower doses of newer, more expensive PIs (such as atazanavir or darunavir) to reach therapeutic levels.
As CNIs and mTOR inhibitors are metabolised by the same enzymatic system, it is rather predictable that drug levels may become astronomical if doses are not substantially reduced. However, there is a concern that in order to achieve reasonable pre-dose levels, total exposure may be reduced, increasing the risk of rejection3.
On the other hand, the use of NNRTIs with a potential to induce CYP3A4, such as efavirenz, may lead to suboptimal immunosuppressive drug concentrations, although with a smaller magnitude than with the use of more potent inducers such as rifampin, phenobarbital, phenytoin or Hypericum perforatum tea or plant extract (St Johns wort). These drug combinations should be avoided if alternative agents can be used. If this is not possible, close monitoring of plasma HIV RNA, with or without antiretroviral dosage adjustment and therapeutic drug monitoring, may be warranted13.
Unlike PIs and NNRTIs, NRTIs do not undergo hepatic transformation through the CYP metabolic pathway. Some, however, do have other routes of hepatic metabolism13. The integrase inhibitor raltegravir has high antiretroviral efficacy and no significant interactions with immunosuppressive agents because of its lack of effect on CYP3A4 and has been successfully used in some cases1. Pretransplant conversion to a cART regimen using raltegravir rather than boosted PI will significantly reduce the potential for interactions and ease postransplant management.
However, these regimens in Portugal are on average EUR 2,600 more expensive yearly than a comparable boosted PI-based regimen: abacavir/lamivudine/atazanavir/ritonavir EUR 30.64/day versus abacavir/lamivudine/raltegravir EUR 37.93/day for average doses (prices from September 2011; data provided by Dr João Rijo, Pharmaceutical Department, Centro Hospitalar de Lisboa Ocidental). Maraviroc-based regimens, likewise free of CYP 3A4 interactions and with the abovementioned attractive immunomodulatory potential, pose similar pharmacoeconomic constraints.
The CYP450 system and P-gp are also involved in the metabolism and elimination of glucocorticosteroids, CNIs and mTOR inhibitors28:
– Glucocorticoids are substrates of CYP3A4 and P-gp. PI inhibit metabolism of glucocorticoids, increasing their plasma concentration and clinical effects, so doses may need to be reduced accordingly20,29. Glucocorticoids may also be inducers of CYP3A4, reducing plasma levels of co-administered PI6.
Patients on glucocorticoids steroids often take ranitidine or proton inhibitors, which can reduce intestinal absorption of the PI atazanavir (very dependent on a low gastric pH) and, therefore, its plasma concentration. This undesirable side effect does not occur with the PI ritonavir2.
– MMF and azathioprine are not metabolised by the CYP450 system or transported by P-gp, so interactions with cART medications are less of an issue6 and they are considered safe immunosuppressants in HIV-positive patients, although only MMF displays antiretroviral activity. However, diarrhoea, a wellknown MMF side effect, may hinder the use of MMF in clinical practice, since it can be added to the diarrhoeal effects of antiretroviral drugs and the disease itself in these patients4.
– CNIs and mTOR inhibitors are substrates and inhibitors of CYP3A4 and P-gp. Administration of these drugs with PIs (namely ritonavir) has the potential to delay elimination and markedly increase blood concentrations of both drugs30,31. Bioavailability is also increased. On average, only 25% of the standard dose of ciclosporin is required if administered concomitantly with PIs32.
As there is a great deal of interindividual variability in patients on NNRTIs, therapeutic concentrations of immunosuppressants such as CNIs and mTOR inhibitors should be monitored routinely, with dosage adjustments made as necessary6. Efavirenz can markedly induce CYP3A4 activity, increasing drug metabolism and leading to decreased plasma drug levels2.
There is evidence that high sirolimus blood levels associated with ciclosporin contribute to the thrombotic microangiopathy pathogenesis, a proven risk factor for thrombotic microangiopathy development in HIV-positive patients4. Cases of haemolytic-uraemic syndrome associated with sirolimus have been reported, possibly resulting from reduced VEGF (related with vascular endothelium viability maintenance) expression induced by sirolimus33.
It is important to pay special attention to other antimicrobiological prophylaxis needs common to other kidney transplant recipients, but which are particularly relevant to HIV-positive individuals. These include Pneumocystis jirovecii prevention with cotrimoxazol or atovaquone and cytomegalovirus with valganciclovir. Attention must also be placed on coinfection with polyomavirus, Epstein-Barr virus, Toxoplasma gondii or human herpes virus-8 (HHV-8).
Noteworthy is the elevated potential of HHV-8 to induce Kaposis sarcoma in this susceptible population32.
SPECIAL SITUATION: PATIENTS WITH HIV-2 INFECTION
Unlike what is observed in HIV-1, standard care in HIV-2 management relies mainly on data from small cohort studies and case series, theoretical assertions, and parallels with HIV-1 therapeutics. HIV-2 infection occurs mainly in West Africa and among here Guinea-Bissau has one of the highest rates of HIV-2 infection35. HIV-2 transmission routes are the same as those for HIV-1, but HIV-2 virus has a lower infectivity36.
As recently described by Ferreira et al., renal disease is not frequent in HIV-2-infected patients, and, when present, is probably not directly associated with HIV infection37.
In comparison to HIV-1, more patients with HIV-2infection present as long-term nonprogressors or slow progressors. Although this could be used to argue for a later CD4 T-cell driven initiation of cART, it has been demonstrated that immunological recovery on therapy could be slower in HIV-2 than HIV-1patients38 and excessive delay in initiating cART may carry negative long-term immunological consequences39. Clinical trials of cART in HIV-2 are scarce compared to the ones available to HIV-1, possibly related to low prevalence and geographic distribution constraints39.
Antiretroviral susceptibility can differ significantly between HIV-1 and HIV-2, such as that HIV-2is intrinsically resistant to two of the major classes of antiretroviral drugs: NNRTIs and fusion inhibitors. Considering the class of PI, indinavir, saquinavir, lopinavir and darunavir are the most efficient molecules in HIV-2supression21.
The authors recently reported a successful case of kidney transplantation in an HIV-2positive patient, the first described in the literature40.
CONCLUSIONS
Unlike cardiac and hepatic transplantation for which there is no other alternative to life support, patients with ESRD have dialysis as an alternative renal replacement therapy. This fact must always be weighed in the individual assessment of potential risks and benefits.
Note that some HIV-positive patients have been stable on dialysis for over 10 years, showing steady health state. Conversely, there is no long-term experience in HIV-positive kidney transplant recipients.
However, kidney transplantation is already an option for selected HIV-infected patients. Further studies are required to identify the optimal choice of immunosuppressive therapy in this group of patients.
REFERENCES
1. Trullas JC, Cofan F, Tuset M, et al. Renal transplantation in HIV- infected patients: 2010 update. Kidney Int 2011;79:825-842 [ Links ]
2. Frassetto LA, Tan-Tam C, Stock PG. Renal transplantation in patients with HIV. Nat Rev Nephrol 2009;5:582-589 [ Links ]
3. Stock PG, Barin B, Murphy B, et al. Outcomes of kidney transplantation in HIVinfected recipients. N Engl J Med 2010;363:2004-2014 [ Links ]
4. Moreno CN, Siqueira RC, Noronha IL. Kidney transplantation in HIV infected patients. Rev Assoc Med Bras 2011;57:98-104 [ Links ]
5.http://www.spnefro.pt/comissoes_gabinetes/Gabinete_registo_2010/default.asp
6.Berns JS, Kasbekar N. Highly active antiretroviral therapy and the kidney: an update on antiretroviral medications for nephrologists. Clin J Am Soc Nephrol 2006;1:117-129 [ Links ]
7. Gupta SK, Eustace JA, Winston JA, Bodydstun II, et al. Guidelines for management of chronic kidney disease in HIV-infected patients: recommendations of the HIV Medicine Association of the Infectious Disease Society of America. Clin Infect Diseases 2005;40:1559-1585 [ Links ]
8. Swanson SJ, Kirk AD, Ko CW, Jones CA, Agodoa LY, Abbot KC. Impact of HIV seropositivity on graft and patient survival after cadaveric renal transplantation in the United States in the pre highly active antiretroviral therapy (HAART) era: an historical cohort analysis of the USRDS. Transpl Infect Dis 2002;4:144-147 [ Links ]
9.Ahuja TS, Borucki M, Funtanilla M, Shahinian V, Hollander M, Rajaraman S. Is the prevalence of HIV-associated nephropathy decreasing? Am J Nephrol 1999;19:655-659 [ Links ]
10. Lucas GM, Eustace JA, Sozio S, Mentari EK, Appiah KA, Moore RD. Highly active antiretroviral therapy and the incidence of HIV-1 associated nephropathy: a 12-year cohort study. AIDS 2004;18:541-546 [ Links ]
11. Ross MJ, Klotman PE, Winston JA. HIV-associated nephropathy: case study and review of the literature. AIDS Patient Care STDS 2000;14:637-645 [ Links ]
12. Abbot KC, Swanson SJ, Agodoa LY, Kimmel PL. Human immunodeficiency virus infection and kidney transplantation in the era of highly active antiretroviral therapy and modern immunosuppression. J Am Soc Nephrol 2004;15:1633-1639 [ Links ]
13. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services 2011;1-67 [ Links ]
14. Thompson MA, Aberg JA, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA 2010; 304: 321-333 [ Links ]
15. Reid A, Stöhr W, Walker AS, et al. Severe renal dysfunction and risk factors associated with renal impairment in HIV-infected adults in Africa initiating antiretroviral therapy. Clin Infect Dis 2008;46:1271-1281 [ Links ]
16. Landin L, Rodrigues-Perez JC, Garcia-Bello MA, et al. Kidney transplants in HIV-positive recipients under HAART. A comprehensive review and meta-analysis of 12 series. Nephrol Dial Transplant 2010;25:3106-3115 [ Links ]
17. Roland ME, Barin B, Carlson L, et al. HIV-infected liver and kidney transplant recipients: 1-and 3-year outcomes. Am J Transplant 2008;8:355-365 [ Links ]
18.Kumar MS, Sierka DR, Damask AM, Fyfe B, et al. Safety and success of kidney transplantation and concomitant immunosuppression in HIV-positive patients. Kidney Int 2005;67:1622-1629 [ Links ]
19. Carter JT, Meicher ML, Carlson LL, et al. Thymoglobulin-associated CD4+ T-cell depletion and infection risk in HIV-infected renal transplant recipients. Am J Transplant 2006;6:753-760
20. Izzedine H, Launay-Vacher V, Baumelou A, et al. Antiretroviral and immunosuppressive drug-drug interactions: an update. Kidney Int 2004;66:532-541 [ Links ]
21. Heredia A, Margolis D, Oldach D, et al. Abacavir in combination with the inosine monophosphate dehydrogenase (IMPDH)-inhibitor mycophenolic acid is active against multidrug-resistant HIV-1. J Acquir Immune Defic Syndr 1999;22:406-407 [ Links ]
22. Latinovic O, Kuruppu J, Davis C, Le N, Heredia A. Pharmacotherapy of HIV-1 infection: focus on CCR5 antagonist maraviroc. Clin Med Ther 2009;1:1497-1510 [ Links ]
23. Fischereder M, Luckow B, Hocher B, et al. CC chemokine receptor 5 and renal-transplant survival. Lancet 2001;357:1758 [ Links ]
24. Trullas JC, Cofan F, Cocchi S, et al. Effect of thymoglobulin induction on HIV-infected renal transplant recipients: differences between HIV-positive and HIV-negative patients. AIDS Res Hum Retroviruses 2007;23:1161-1165 [ Links ]
25. Locke JE, Montgomery RA, Warren DS, et al. Renal transplant in HIV-positive patients: long-term outcomes and risk factors for graft loss. Arch Surg 2009;144:83-86. [ Links ]
26. Stock PG, Roland ME, Carlson L, et al. Kidney and liver transplantation in human immunodeficiency virus-infected patients: a pilot safety and efficacy study. Transplantation 2003;76:370-375 [ Links ]
27. Stock PG, Roland ME. Evolving clinical strategies for transplantation in HIV-positive recipient. Transplantation 2007;84:563-571 [ Links ]
28. Lo A, Burckart GJ. P-glycoprotein and drug therapy in organ transplantation. J Clin Pharmacol 1999;39:995-1005 [ Links ]
29. Maat MM, Ekhart GC, Huitema AD, Koks CH, Mulder JW, Beijnen JH. Drug interactions between antiretroviral drugs and comedicated agents. Clin Pharmacokinetic 2003;42:223-282 [ Links ]
30.Jain AK, Venkataramanan R, Shapiro R, et al. The interaction between antiretroviral agents and tacrolimus in liver and kidney transplant recipients. Liver Transpl 2002;8:841-845 [ Links ]
31.Frassetto L, Thai T, Aggarwal AM, et al. Pharmacokinetic interactions between cyclosporine and protease inhibitors in HIV+ subjects. Drug Metab Pharmacokinet 2003;18:114-120 [ Links ]
32. Stock P, Roland M, Carlson L, et al. Solid organ transplantation in HIV-positive patients. Transplant Proc 2001;3:3646-8 [ Links ]
33. Sartelet H, Toupance O, Lorenzato M, et al. Sirolimus-induced thrombotic microangiopathy is associated with decreased expression of vascular endothelial growth factor in kidneys. Am J Transplant 2005;5:2441-2447 [ Links ]
34. Weigert AL, Pires A, Adragão T, et al. Human herpes virus-8 serology and DNA analysis in recipients of renal allografts showing Kaposis sarcoma and their respective donors. Transplant Proc 2004;36:902-904 [ Links ]
35. Clavel F, Mansinho K, Chamaret S, et al. Human immunodeficiency virus type 2 infection associated with AIDS in West Africa. N Engl J Med 1987;316:1180-1185 [ Links ]
36. Brown P. HIV-2: slower, still deadly. World AIDS 1992;22 [ Links ]
37. Ferreira AC, Carvalho D, Carvalho F, Galvão MJ, Nolasco F. Renal pathology in Portuguese HIV-infected patients. Port J Nephrol Hypert 2011;25:275-283 [ Links ]
38. Matheron S, Damond F, Benard A, et al. CD4 cell recovery in treated HIV-2 infected adults is lower than expected: results from the French ANRS CO5 HIV-2 cohort. AIDS 2006;20:459-462 [ Links ]
39. Peterson K, Jallow S, Rowland-Jones SL, et al. Antiretroviral therapy for HIV-2 infection: recommendations for management in low resource settings. AIDS Res Treat 2010. http://www.hindawi.com/journals/art/2011/463704/2011 [ Links ]
40.Natário A, Rodrigues B, Matias P, et al.Renal transplantation in a HIV-2 positive recipient – a case report. XII Congresso Brasileiro de Transplantes. Belém, Brasil, 2011. [ Links ]
Dr Ana Natário
Department of Nephrology
Centro Hospitalar Setúbal
Rua Camilo Castelo Branco
2910-446 Setúbal, Portugal
E-mail:ananatario@hotmail.com
Conflict of interest statement. None declared.
Received for publication:20/11/2011
Accepted in revised form:01/02/2012














