Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista Portuguesa de Imunoalergologia
versão impressa ISSN 0871-9721
Rev Port Imunoalergologia vol.22 no.2 Lisboa jun. 2014
ARTIGO ORIGINAL
Parietaria judaica pollen: Aerobiology and allergenicity
Pólen de Parietaria judaica: Aerobiologia e alergenicidade
Raquel Sousa1, Helena Ribeiro1, Ana Cruz2, Laura Duque1, Ilda Abreu1,3
1 Grupo Ambiente do Centro de Geologia da Universidade do Porto
2 Serviço de Patologia Clínica, Laboratório de Imunologia do Centro Hospitalar de Vila Nova de Gaia
3 Departamento de Biologia da Faculdade de Ciências da Universidade do Porto
ABSTRACT
Introduction: Parietaria judaica pollen is able to induce severe allergic symptoms, mainly due to its high pollen output and the long persistence in the atmosphere. Aim: We intended to study the aerobiology and allergenic proteins of Parietaria judaica pollen collected in an urban area of the Porto region. Methods: We adopted aerobiological and immunological techniques to study the annual airborne pollen concentrations from 2003 to 2012 and the immunoreactivity of P. judaica pollen extracts against 21 sensitized patient sera. Results: Parietaria spp. pollen is pre sent in the atmosphere of Porto all year long, representing 17% of the total pollen spectrum. The highest airborne pollen concentrations were mainly found from April-August. From the selected patients with pollen allergy, 34.2% showed sensitization to Parietaria spp. pollen extracts. Among these, 84.5% were only positive to Parietaria judaica and 8.5% to its counterpart Parietaria officinalis. The most prevalent protein bands of P. judaica pollen extracts when ted against allergic patient sera were those around 55 kDa, 36-30 kDa, 14 kDa and 12-11 kDa. An anti-Par j 1/Par j 2 immunoblot revealed two protein bands around 12 kDa and two possible glycoprotein bands of around 22 and 18 kDa were also showed in the P. judaica pollen extracts. Conclusions: Parietaria spp. is the most abundant pollen type in the atmosphere of Porto and its highest concentrations are found at the first sunlight hours. P. judaica in particular sensitizes a considerable fraction of pollen-sensitive individuals and several reactive protein bands were recognized by P. judaica sensitive IgE sera. This pollen appears to be an important source of weed -sensitization in this region.
Keywords: Aerobiological profile, allergens, Parietaria judaica, pollen, sensitization.
RESUMO
Introdução: O pólen de Parietaria judaica é capaz de induzir doença respiratória alérgica, principalmente devido à elevada produção de pólen e persistência na atmosfera. Objectivo: Estudámos a aerobiologia e as proteínas potencialmente alergénicas do pólen de P. judaica recolhido na área urbana do Porto. Métodos: Adoptámos técnicas aerobiológicas e imunológicas de forma a estudar o espectro polínico entre 2003 e 2012 e o perfil reactivo de extractos proteicos de pólen de P. judaica quando incubados com 21 soros de indivíduos alérgicos a pólen, previamente seleccionados. Resultados: O pólen de Parietaria spp. Está presente na atmosfera praticamente todo o ano, representando 17% do espectro polínico total. A concentração de pólen mais elevada foi verificada entre Abril e Agosto. Entre indivíduos alérgicos a pólen, 34,2% mostrou sensibilização a extractos polínicos de Parietaria spp. Destes, 84,5% mostraram apenas reacção positiva a Parietaria judaica e 8,5% a Parietaria officinalis. Após incubação com os soros seleccionados, os immunoblots de extracto de pólen de P. judaica apresentaram bandas proteicas reactivas mais prevalentes de aproximadamente 55 kDa, 36-30 kDa, 14 kDa e 12-11 kDa. A incubação de um immunoblot de extracto de pólen de P. judaica com anti-Par j 1/Par j 2 revelou a existência de duas bandas proteicas aproximadamente de 12 kDa.
Foram também detectadas duas bandas proteicas glicosiladas no extrato de P. judaica, uma de cerca de 22 kDa e outra aproximadamente de 18 kDa. Conclusões: O pólen de Parietaria spp. é o mais abundante na atmosfera do Porto, surgindo em concentrações mais elevadas durante as primeiras horas de luz solar. O pólen de P. judaica sensibiliza uma fracção considerável dos doentes com alergias respiratórias e os soros de doentes sensibilizados demonstraram marcação de vários componentes de ligação a IgE. Assim, o pólen de P. judaica parece ser uma importante causa de sensibilização a herbáceas na região do Porto.
Palavras-chave: Alergénios, Parietaria judaica, perfil aerobiológico, pólen, sensibilização.
INTRODUCTION
Pollen related respiratory allergies, or as common known pollinosis, is a general health problem worldwide, affecting life quality of Earth inhabitants.
They are associated to an allergic response of patients to the pollen grains of several trees, grasses and weeds.
Sensitization, which is the exposure to allergens, starts the immune response in allergy by eliciting the production of immunoglobulin E antibodies.
Pollen grains are biological structures produced by higher plants. They are transported from the anthers to the stigma of the same flower or of a different flower of the same species by various abiotic and/or biotic agents.
This dependence on environmental conditions for successful fertilisation led to anemophilous plants developing compensatory mechanisms, such as the release of large amounts of airborne pollen and production of more aerodynamic pollen grains, making dispersal easier. These characteristics, associated to the existence of allergens in the pollen wall are the main cause triggering respiratory allergic reactions. To estimate the prevalence of pollen allergy in people who present such symptoms and to measure allergen exposure, clinicians carry out sensitization studies on a regular basis.
The development of aerobiological studies is important for the elaboration of pollen calendars, after several years of monitoring, estimating the date for the flowering season of plants. This know ledge is useful for clinicians in order to adequate the standard skin prick test battery with extracts to the regional pollen spectrum, for effective immunotherapy.
It can also be helpful for patients in taking prophylactic measures such as the planning of outdoor activities, contributing to an improvement on their life quality.
In Portugal, some studies have been conducted on the type and concentration of airborne pollen grains in the atmosphere of several cities1-5as well as its relationship with meteorological factors6. Also, the classification of allergenic pollen types, through immunological studies7,8 associating the regional pollen exposure with allergic respiratory hospital admissions7, has been performed.
Pollen allergy has a remarkable clinical impact all over Europe, and there is considerable evidence suggesting that respiratory pollen allergic diseases are increasing in both prevalence and severity in most industrialized and developing countries9.
The plants of Urticaceae family that comprise the genus Parietariaare dicotyledonous weeds and have been described as the most widespread allergenic plants in the Mediterranean area10. Its pollen is responsible for many cases of severe pollinosis mainly due to its high pollen output and the long persistence in the atmosphere10,11.
Parietaria judaica and Parietaria officinalis are the most common allergenic species of this genus and are highly cross-reactive12. Parietaria judaicaor wall pellitory (Figures 1a, 1b) has very strong allergenic properties having a particular allergological interest13. It is a sprawling, many-branched, bushy perennial weed with delicate reddish stems. It is wind-pollinated and grows spontaneously on neglected areas, along roadsides, on walls, inside riverside forests and hedges, reaching from 30 to 100 cm in high. The leaves are 3 to 12 cm long and oval in shape, with hairs on the veins on the lower surface. The inconspicuous green stalkless flowers are clustered in the leafaxils.
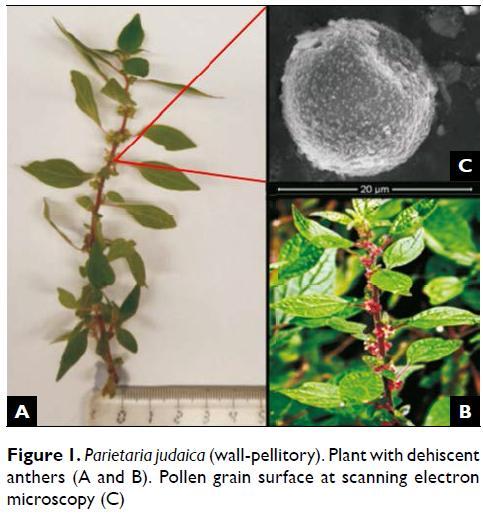
P. judaica pollen contains at least nine allergens identified of which two major allergens – Par j 1 of 14.7 kDa and Par j 2 of 11.3 kDa that have already been biochemically characterized and molecular cloned by different immunochemical methodologies14,15,16. They were reported to belong to a family of glycoproteins known as non-specific lipid transfer proteins (LTP)13,17,18. Their recombinant proteins showed a prevalence of 95% and 82% among P. judaica-sensitive patients19,20. The sequence analysis of the cDNA expression library has shown that Par j 1 allergen (Par j 1.0102) contains 176 amino acids with an amino-terminal region of 37 amino acids and a mature processed protein of 139 amino acids with a molecular weight of 14,729 kDa19. The other major allergen of P. judaicapollen, Par j 2 (Par j 2.0101), was also sequenced and consists of a polypeptide of 133 amino acids with an amino-terminal signal peptide of 31 amino acids giving a mature processed protein of 11,344 kDa20. More recently, a profilin of P. judaica – Par j 3 – was identified as having a molecular mass similar to Par j 1 and Par j 2, within the range of 14 kDa21. In our investigation, we sought to study the aerobiology and allergenic proteins of Parietaria judaica pollen collected in the Porto region.
MATERIAL AND METHODS
Study area
This study was conducted in the Porto region, the second largest Portuguese city, located in the Northwest of Portugal (41º11 N, 8º39W) being limited by the Atlantic Ocean and the Douro River. It presents a Mediterranean climate according to the Köppen climate classification, however, an Atlantic influence can be observed.
Temperature is mild; January is the coldest month and July the hottest. The average minimum temperature is 10±0.7ºC and the average maximum 19±0.6ºC. Annual mean relative humidity ranges between 75 and 80%, and rainfall is mainly concentrated in winter and spring22.
Aerobiological monitoring
Airborne pollen monitoring was continuously performed from 2003 to 2012, using a 7-day Hirst–type volumetric trap (Burkard Manufacturing Co., U.K.) set on the roof of the Faculty of Sciences in Porto, 20 m above ground level, and calibrated to sample air at 10 L per minute. Pollen grains were trapped on a Melinex tape coated with silicone oil, which was then cut into daily segments and mounted on slides with a mounting media of glycerol jelly with fuchsine. The daily and hourly mean concentration of Parietaria spp. pollen was estimated using an optical microscope (DMLB, Leica) with x400 magnification along 4 full lengthwise traverses. Pollen counts were expressed as the sum of the number of pollen grains per cubic meter of air for a 24 -hour period. P. judaica pollen is oblate (circular) usually tri-zonoporate and its polar axis commonly ranges from 12 to 16 μ m. The external layer named exine is thin, psilate to scabrate with spinulas uniform and densely distributed over the surface (Figure 1c).

The intradiurnal airborne pollen concentration was determined, for each year, from the hourly values of atmospheric concentration registered during the main pollen season23. For this, the days when atmospheric pollen concentrations were superior to the third quartile of the main pollen season concentration and no precipitation occurred were selected. Since the hourly counts vary widely interdaily and interannually, the values were expressed in percentage.
The main pollen season was defined using a non-linear logistic regression model fitted to the values of the accumulated sum of the daily airborne pollen concentration.
After adjusting the model to each year, a one-sided t-test was used at the 5% level, in order to estimate the beginning and ending dates of the main pollen season. These dates correspond to the thresholds where the daily difference between the pollen emission model and its superior and inferior asymptotes were significant24.
Pollen data did not present normal distribution, so data was transformed using Log (yi+1), where yi corresponds to each pollen value registered, once values were very dispersed and some were null. The relationship between the main meteorological parameters (mean, mini mum and maximum temperature and relative humidity) and the daily pollen concentration was calculated using a Pearson correlation coefficient.
Sensitization data
Data from allergic patients sera presenting specific IgE levels to pollen extracts, performed by Immuno-CAPTM FEIA test (Phadia AB), were obtained from a central hospital in Porto region between 2007 and 2012.
This study was performed only with anonymous files of sensitization test results due to confidential policies and therefore information regarding gender, sex or age was unavailable.
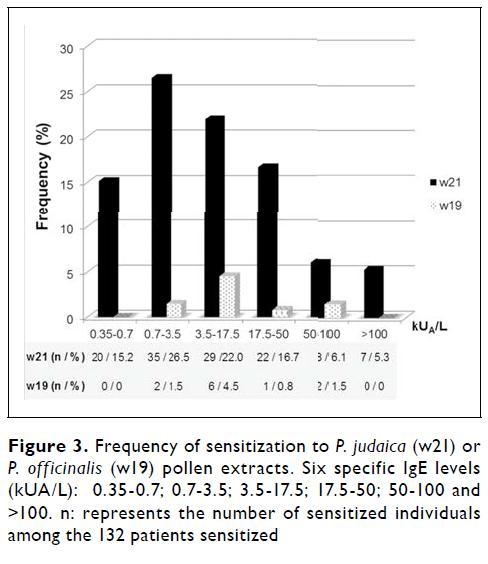
Patients presenting specific IgE levels to Parietaria judaica (w21) or Parietaria officinalis(w19) and both pollen extracts were accounted by different levels of specific IgE recognition: 0.35 -0.7 kUA/L; 0.7 -3.5 kUA/L; 3.5 -17.5 kUA/L; 17.5 -50 kUA/L; 50 -100 kUA/L; >100 kUA/L.
Biochemical and Immunological studies
Pollen samples
Anthers were collected during Parietaria judaicaflowering season and after removal of other plant parts, they were dried at 27°C, gently crushed and the pollen thus released was passed through different grades of sieves to obtain pure pollen. Pollen samples were then stored at –20°C.
Patient sera Twenty-one random Parietaria-sensitive patients with clinical history of respiratory allergy and presenting a positive response to skin–prick tests (SPT) using Parietaria judaica pollen extracts were chosen. A positive SPT was considered when allergen ring diameter was 3 mm or greater after subtraction of the negative control, recorded at 15 minutes post-injection. Histamine (10 mg/mL) and saline solutions of glycerin were used as the positive and negative controls, respectively. Blood from those patients was collected by venipuncture, sera were separated from the whole blood and allergen specific IgE levels were measured by ImmunoCAPTM FEIA test (Phadia). The chosen sera had specific IgE levels against Parietaria judaicapollen extracts (w21) between 0.35 and >100 kUA/L. One serum from a patient with low total IgE levels and negative to Parietaria judaica pollen was also used as a negative control (NC). This study was performed only with anonymous files due to confidential policies and therefore information regarding gender, sex or age was unavailable.
Pro tein extraction and quantification P. judaica dry pollen was suspended in 1:20 (w/v) phosphate saline buffer at pH 7.4 at 4°C. Soluble proteins were extracted in the same buffer by continuous stirring for 4 h.
The suspension was then centrifuged at 13,200 rpm for 30 min at 4°C. The supernatant was filtered through a nitrocellulose 0.45 μm Millipore syringe filter and centrifuged once again. The soluble protein content of crude pollen extracts was colorimetrically quantified using the Coomassie Protein Assay Reagent.
SDS-Polyacrylamide Gel Electrophoresis and staining SDS-PAGE was performed in 12.5 % polyacrylamide gels under reducing conditions. The polypeptides of pollen crude extracts were visualized by Coomassie Brilliant Blue R-250 staining. The molecular weights of protein bands were estimated by comparison with a protein marker (Fermentas now Thermo Scientific). The antigenic profile was studied using a Molecular Imager GS800 calibrated densitometer and Quantity One 1 -D Analysis, v 4.6 software (Bio-Rad Laboratories).
Glycosylated protein detection Total carbohydrate detection of P. judaica protein extract transferred to a nitrocellulose membrane was performed using an ECLTM Glycoprotein Detection System RPN2190 (GE Healthcare, Amersham), following the manufacturers instructions. This system allows labeling of a carbohydrate moiety with biotin through the periodate oxidation step and later detection with streptavidin-HRP and enhanced chemiluminescence.
Immunoblot and inhibition experiments Protein bands separated by SDS-PAGE were electrophoretically transferred on a blot apparatus (TE22 Mighty Small Transfer Unit, GE Healthcare, Amersham) to a nitrocellulose membrane (Protran, Whatman® Schleicher and Schuell, Germany). Transfers were carried out in a solution of 192 mM glycine, 25 mM Tris and 20% methanol during 2 h at 200 mA. The membranes were saturated during 1 h with 5% non-fat dry milk, 0.1% goat serum in TBS-T (20 mM Tris, 150 mM NaCl with 0.1% Tween 20) and incubated overnight at 4 °C with P. judaica allergic patient sera (1:10); polyclonal serum raised against recombinant maize profilin (ZmPRO3) (1:4000), expressed in E. coli and produced in a New Zealand white rabbit25; Lol p 1 (1:200) or Par j 1/Par j 2 (1:100), used as reference material, purified from rye grass pollen extract by a combination of ion exchange chromatography and gel filtration26. After washing, blots were probed with mouse anti–human IgE-HRP or with goat anti-rabbit IgG-HRP during 1 h at 4.ºC. The reaction was revealed using peroxidase ECL substrate and exposed to ECL–Hyperfilm (GE-Healthcare). The chemiluminescent signal was exposed to AGFA medical X-ray film, developed by Fuji medical film processor model FPM 100A, and the antigenic profile was quantified as described above.
SDS-PAGE resolved P. judaica pollen proteins were transferred onto nitrocellulose membranes. After blocking, two blots were kept overnight at 4.ºC with aliquots of patient serum only sensitized to P. judaica pollen extract with saturating buffer as the control experiment (1:5 v/v) or pre -incubated with P. judaica pollen extract as inhibitor. Bound IgE was detected as described previously.
RESULTS
Aerobiological monitoring
Considering the period between January 2003 and September 2012, Parietaria spp. pollen was the most abundant type in the atmosphere of Porto and constitutes nearly 17% of the total pollen spectrum. The annual pollen concentration ranged from 654 pollen grains in 2003 to 2,921 pollen grains per cubic meter of air in 2007 (Table 1).
The highest Parietaria spp. airborne pollen concentrations were mainly found between April and August, being the maximum airborne concentration usually between May and July. The average main pollen season was of 13±4.3 weeks, being the longest main pollen season recorded in 2004, with 153 days and the shortest in 2012, with 62 days. The average airborne pollen peak levels attained within a 24h-period was 50 pollen.m-3 of air, being the highest values observed in 2007, with 110 pollen.m-3 of air, and the lowest in 2003, with 18 pollen.m-3 of air (Table 1; Figure 2A). Concerning intradiurnal variation, the highest airborne pollen concentrations were recorded during the morning period. Parietariaairborne pollen started to increase at first day hours (7 a.m.) reaching a maximum value between 9-11 a.m. that corresponded on average to 10% of its daily total. A similar concentration, around 3%, during the other hours of the day was observed (Figure 2B).
Overall correlations between Parietaria spp. Airborne pollen concentration and the meteorological factors showed positive correlations with the thermal parameters and negative with precipitation and relative humidity.
Consistent correlations along the studied years were observed for maximum and mean temperature (Table 2).
Sensitization assessment
Out of 415 patients presenting specific IgE levels to any pollen, previously evaluated by SPT and presenting positive reaction to pollen extracts, 142 patients (34.2%) presented specific IgE levels to Parietaria spp. pollen extracts.
Among these, 84.5% (n=120) were only positive to Parietaria judaica (w21), 8.5% (n=12) to Parietaria officinalis(w19) and 7.0% (n=10) to both pollen types (w21 and w19).
Among the specific IgE levels, the highest percentage of patients had 0.7 -3.5 kUA/L for w21 (Figure 3). Extreme IgE affinity (>100 kUA/L) was only achieved by P. judaica sensitized patients. Demographic data were not available due to confidentiality policy.
Immunoreactivity of pollen extracts, inhibition experiment
SDS-PAGE protein profiles of P. judaica pollen extracts are presented in Figure 4. The resolved protein fractions on SDS-PAGE ranged from 100 to 10 kDa, being the most noticeable bands around 55, 45, 36, 30, 22, 18, 14, 12 and 11 kDa.
The reactivity of the protein bands from P. judaicapollen extracts was assayed by immunoblotting. The specific IgE binding fractions incubated with P. judaica sensitive patients sera are shown in Figure 4. Among the twenty-one tested patient sera, four main groups of common reactive bands were observed: one band around 55 kDa (n=15, 66.7%), one group with two bands of 36 and 30 kDa (n=13, 61.9%), another group of two bands with 22 and 18 kDa (n=8, 38.1%) and a fourth group composed by three bands of 14 kDa (n=14, 66.7%) and a pair of 12 and 11 kDa (n=18, 85.7%).
To ensure that a serum is specifically sensitive to Parietaria judaica pollen extracts an inhibition experiment was conducted. Pre -incubation of P. judaica pollen protein extracts with a serum sensitized to P. judaicapollen inhibited completely the patient serum sensitive to P. judaica.
Allergen-like proteins and glycosylated protein detection
To identify Par j1/Par j2 -like proteins in P. judaica pollen extracts it was also carried out an anti-Par j 1/Par j 2 immunoblot, which revealed two protein bands of 12 and 11 kDa.
To analyze the similarity of P. judaica pollen proteins to some relevant allergens of non-taxonomically-related proteins an immunoblot using ZmPRO3, a profilin from Zea mays, and Lol p 1, a major grass pollen allergen of Lolium perenne were performed. After the incubation with ZmPRO3 and Lol p 1, P. judaica immunoblots revealed one protein band around 14 kDa and another band around 32 kDa, respectively.
We also investigated the presence of glycosylated proteins on P. judaica pollen extracts. After total carbohydrate labeling, two bands were present in P. judaica extract profile, one around 22 kDa and another of around 18 kDa.
DISCUSSION
The weed Parietaria spp., mainly P. judaica, is considered one of the most relevant airborne allergens of the Mediterranean area. So, the present study performed aerobiological monitoring in a Northern Portuguese coastal city to assess the IgE affinity to P. judaica pollen protein extracts of sensitized individuals.
Atmospheric pollen concentration varies from region to region, depending on its geographic characteristics as different climatic areas present different vegetation. In the Porto region, Parietaria spp. is the most abundant pollen present in the atmosphere. The same pattern was also found in Lisbon (Portugals capital), but was different from Coimbra, Évora and Portimão where Urticaceae that includes Urtica spp. and Parietaria spp. are the fourth most representative pollen types1. This pollen is present all year long in the atmosphere of Porto; this can be related to the fact that Parietaria spp. is a weed species that is able to grow and flower along the year, and also pre sents light and aerodynamically shaped pollen (small size 12-16 μm) that can resuspend in the air very easily. Consequently, airborne pollen can be found in the atmosphere outside of the main pollen emission season. This sustains several implications in allergenic sensitization, observed mainly during the spring-summer months. In fact, some days presented airborne pollen levels of Parietaria spp. superior to 30 pollen grains.m-3 per day and in some years even superior to 60 pollen grains.m-3 per day that are considered, according to the Portuguese Aerobiology Network, moderate and high risk for allergenic reactions.
The high Parietaria spp. airborne pollen concentrations are found during the sunlight hours. This pattern is common to a wide range of pollen types4, since the anthesis and pollen dispersion occurs mainly during daylight. Likewise, the increase in pollen concentration during the first hours of sunlight coincides with a period of thermal inversion, increase in wind velocity and decrease in relative humidity that facilitates anther dehiscence, pollen emission and dispersion27. Apart from the high airborne pollen peak reported at 10h, Parietaria spp. pollen was presented within an almost constant threshold during the other hours of the day. This fact is important because it can contribute to the exacerbation of the allergic symptoms due to the cross-reactivity with other pollen types also present in high concentrations in the atmosphere.
Consistent correlations between airborne pollen concentration and the meteorological parameters were only observed for temperature. This is in accordance with the common knowledge that the heat parameters have a positive influence on airborne pollen concentration, since temperature favors pollen release. Regardless of non-significant, the influence of precipitation was always negative and its effect is associated with atmosphere washing, which can have a positive effect on decreasing pollen allergy outbreaks.
In the present study, we evaluated the prevalence of sensitization to Parietaria spp. among 415 pollen sensitive individuals based on the IgE serum levels measured. About 34.2% had specific IgE to Parietaria spp. from which 84.5% specifically and only to P. judaica. This pollen species also seems to be the only weed able to induce extreme IgE recognition in all pollen sensitive patients studied (data not shown). Sensitization, evaluated through skin–prick tests, to this pollen extract has also been described in several studies in other Mediterranean areas. In Central Greece, it was reported a prevalence of 10.3%28; Gioulekas et al.29 reported 15.3% in Northern Greece. In Spain, it was verified that 14% of the population with respiratory allergy symptoms was sensitized to P. judaica pollen30, and in Genoa (Italy) among 6800 patients 70.6% showed positive SPT to P. judaica pollen extracts31. In a sensitization study performed in East -central region of Portugal, the authors sought to evaluate aeroallergens sensitizations among individuals suffering from respiratory allergy according to the environment they lived in (urban vs. rural exposure). These authors verified that among the 1096 pollen positive SPT assessed, 29.4% living in urban areas were sensitized to P. judaica pollen contrasting to 14% living in a rural environment32.
In our study, by using a panel of 21 P. judaica sensitive IgE sera, we observed that protein bands around 55 kDa, 36-30 kDa, 14 kDa and 12-11 kDa were preferentially recognized. It was verified that the majority of the 21 IgE sera tested (85.7%) recognized a pair of proteins with 12-11 kDa that may correspond to the specific allergens of P. judaica pollen extracts13. An anti-Par j 1/Par j 2 was used to probe P. judaica whole extracts and the two protein bands of low molecular weight revealed can be the major allergens of Par j 1/Par j 2-like proteins capable of inhibiting up to 95% of the total IgE binding activity of P. judaicapollen extracts33.
Furthermore, we identified two glycosylated proteins around 22 and 18 kDa from P. judaica whole pollen extracts, which do not correspond to the most IgE reactive group of protein bands. Regarding the estimated molecular weights, these glycosylated proteins may be related to allergens around the same molecular weight recognized by 38.1% of the 21 IgE patient sera tested.
By using rabbit polyclonal IgG ZmPRO3 antibody as a probe instead of IgE sera, a protein band of around 14 kDa was recognized and may correspond to a profilin-like protein, a well-know panallergen that has already been described in Parietaria judaicapollen extracts21. In a skin-prick test sensitization study, it was reported that 64.1 % of profilin-sensitive patients were also sensitized to P. judaica pollen, suggesting that this panallergen may share determinant epitopes with a 14 kDaP. judaica pollen protein34. Asturias et al.21 showed a very low content P. judaica profilin when compared to non-related pollen extracts. In our study, 61.9% of IgE sera sensitive to P. judaica reacted to a protein band around 14 kDa that may well correspond to P. judaica profilin.
Regarding other possible cross-reactive proteins, after probing blots with Lol p 1, a group 1 grass allergen, it was identified a single band of 32 kDa that may correspond to an expansin-like protein35 in P. judaica pollen extracts and can be related with the pair of 36-30 kDa identified with the IgE sera sensitive to P. judaica.
CONCLUSIONS
Parietariaspp. is the most abundant pollen type in the atmosphere of Porto, presenting a main pollen season between April and August and attaining airborne levels considered to be moderate and high risk for allergenic reactions. Its airborne pollen concentration is highest at the first sunlight hours and it is positively correlated with ambient temperature.
P. judaica in particular sensitizes a considerable fraction of pollen-sensitive individuals and several reactive protein bands were recognized by P. judaica sensitive IgE sera, which included a pair of proteins with 12-11 kDa that may correspond to the specific allergens of P. judaica pollen extracts, glycosylated proteins of 22 and 18 kDa and a few cross-reactive proteins of 14 kDa and 32 kDa, which may correspond to a profilin-like and an expansin-like proteins, respectively. So, P. judaica pollen is an important source of weed-sensitization in Porto region.
ACKNOWLEDGMENTS
The authors are extremely grateful to Prof. Chris J.
Funding: This work was supported by FEDER funds through the Operational Program Competitiveness Factors (COMPETE) and National funds through FCT – Foundation for Science and Technology in the Project Effects of atmospheric non-biological pollutants on pollen grains (Ref.ª PTDC/AACAMB/102796/2008 and POCI 2010 -2012. Helena Ribeiro benefits from a scholarship (SFRH/BDP/43604/2008) financed by QREN-POPH and FCT.
REFERENCES
1. Caeiro E, Brandão R, Carmo S, Lopes L, Morais-Almeida M, Gaspar A, et al. Rede Portuguesa de Aerobiologia: Resultados de monitorização do pólen atmosférico (2002 -2006). Rev Port Imunoalergologia 2007;15:235-50. [ Links ]
2. Câmara I, Fernandes AM, Câmara R. Monitorização aerobiológica da cidade do Funchal (2003 -2007). Rev Port Imunoalergologia 2009;17:419-34. [ Links ]
3. Fernandes FM , Molina RT, Carvalho LMM. Estudo aerobiológico de Beja (Sul de Portugal). Rev Port Imunoalergologia 2010; 18:419-29. [ Links ]
4. Ribeiro H, Oliveira M, Abreu I. Intradiurnal variation of allergenic pollen in the city of Porto (Portugal). Aerobiologia 2008;24:173-7. [ Links ]
5. Abreu I, Ribe iro H. Allergenic pollen in the city of Porto (Portugal). Allergy 2005;60:1452-3. [ Links ]
6. Ribeiro H, Cunha M, Abreu I. Airborne pollen concentration in the regions of Braga, Portugal, and its relationship wit meteorological parameters. Aerobiologia 2003;19:21-7. [ Links ]
7. Ribeiro H, Oliveira M, Ribeiro N, Cruz A, Ferreira A, Machado H, et al. Pollen allergenic potential nature of some trees species: A multidisciplinary approach using aerobiological, immunochemical and hospital admissions data. Environ Res 2009;109:328-33. [ Links ]
8. Sousa R, Cruz A, Ribeiro H, Abreu I. Impact of urbanization level on Chenopodium album pollen: morphological and immunochemical data. Rev Port Imunoalergologia 2011;19:33-41. [ Links ]
9. DAmato G, Cecchi L, DAmato M, Liccardi G. Urban air pollution and climate change as environmental risk factors of respiratory allergy: An update. J Invest Allerg Clin 2010;20:95-102. [ Links ]
10. Masullo M, Mariotta S, Torrelli L, Graziani E, Anticoli S, Mannino F. Respiratory allergy to parietaria pollen in 348 subjects. Allergol Immunopathol (Madr) 1996;24:3-6. [ Links ]
11. Serafini V. Studies on hay fever with special regard to pollinosis due to Parietaria officinalis. Acta Allergol 1975;11:3-27. [ Links ]
12. Ayuso R, Carreira J, Polo F. Quantitation of the major allergen of several Parietaria pollens by an anti-Par 1 monoclonal antibody-based ELISA – Analysis of cross-reactivity among purified par J 1, Par O 1 and Par M 1 allergens. Clin Exp Allergy 1995;25:993-9. [ Links ]
13. Colombo P, Bonura A, Costa M, Izzo V, Passantino R, Locorotondo G, et al. The allergens of Parietaria. Int Arch Allergy Immunol 2003;130:173-9. [ Links ]
14. Ayuso R, Carreira J, Lombardero M, Duffort O, Peris A, Basomba A, et al. Isolation by Mab Based Affinity-Chromatography of 2 Par J I Isoallergens – Comparison of Their Physicochemical, Immunochemical and Allergenic Properties. Mol Immunol 1993;30:1347-54. [ Links ]
15. Amoresano A, Pucci P, Duro G, Colombo P, Costa MA, Izzo V, et al. Assignment of disulphide bridges in Par j 2.0101, a major allergen of Parietaria judaica pollen. Biol Chem 2003;384:1165-72. [ Links ]
16. Stumvoll S, Westritschnig K, Lidholm J, Spitzanewr S, Colombo P, Duro G, et al. Identification of cross-reactive and genuine Parietaria judaica pollen allergens. J Allergy Clin Immun 2003;111:974-9. [ Links ]
17. Asturias JA, Gomez -Bayon N, Eseverri JL, Martinez A. Par j 1 and Par j 2, the major allergens from Parietaria judaica pollen, have similar immunoglobulin E epitopes. Clin Exp Allergy 2003;33:518-24. [ Links ]
18. Salcedo G, Sanchez-Monge R, Diaz-Perales A, Garcia-Casado G, Barber D. Plant non-specific lipid transfer proteins as food and pollen allergens. Clin Exp Allergy 2004;34:1336-41. [ Links ]
19. Costa MA, Co lombo P, Izzo V, Kennedy H, Venturella S, Cocchiara R, et al. cDNA cloning, expression and primary structure of Par J I, a major allergen of parietaria judaica pollen. FEBS Lett 1994;341:182-6. [ Links ]
20. Duro G, Colo mbo P, Costa MA, Izzo V, Porcasi R, Di Flore R, et al. cDNA cloning, sequence analysis and allergological characterization of Par j 2.0101, a new major allergen of the Parietaria judaica pollen. FEBS Lett 1996;399:295-8. [ Links ]
21. Asturias JA, Ibarrola I, Eseverri JL, Arilla MC, Gonzalez -Rioja R, Martinez A. PCR-based cloning and immunological characterization of Parietaria judaica pollen profilin. J Invest Allerg Clin 2004;14:43-8. [ Links ]
22. Miranda P, Coelho FES, Tomé AR, Valente MA. 20th Century Portuguese Climate and Climate Scenarios. In: Gradiva, ed. FD Santos, K Forbes, R Moita (Eds). Climate Change in Portugal Scenarios, Impacts and Adaptation Measures – SIAM Executive Summary and Conclusions 1st ed. Lisboa; 2001:28-84. [ Links ]
23. Galán C, Torm o R, Cuevas J, Infante F, Domínguez E. Theoretical daily variation patterns of airborne pollen in the South -West of Spain. Grana 1991; 30:201-9. [ Links ]
24. Ribeiro H, Cunha M, Abreu I. Definition of main pollen season using a logistic model. Ann Agr Env Med 2007;14:259-64. [ Links ]
25. Karakesisoglou I SM, Gibbon BC, Staiger CJ. Plant profilins rescue the aberrant phenotype of profilin-deficient Dictyostelium cells. Cell Motil Cytoskeleton 1996;34:36-47. [ Links ]
26. Arilla MC, Ibarrola I, Eraso E, Aguirre M, Martinez A, Asturias JA. Quantification in mass units of group 1 grass allergens by a monoclonal antibody-based sandwich ELISA. Clin Exp Allergy 2001;31:1271-8. [ Links ]
27. Ribeiro H, Oliveira M, Abreu I. Variação horária do pólen de Urticaceae e Poaceae na atmosfera do Porto. Rev Port Imunoalergologia 2008;16:163-73. [ Links ]
28. Anastassakis KK, Chatzimichail A, Androulakis I, Charisoulis S, Riga M, Eleftheriadou A, et al. Skin prick test reactivity to common aeroallergens and ARIA classification of allergic rhinitis in patients of Central Greece. Eur Arch Otorhinolaryngol 2010;267:77-85. [ Links ]
29. Gioulekas D PD, Damialis A, Spieksma F, Giouleka P, Patakas D. Allergenic pollen records (15 years) and sensitization in patients with respiratory allergy in Thessaloniki, Greece. Allergy 2004;59:174–84. [ Links ]
30. Navarro A, Valero A , Julia B, Quirce S. Coexistence of asthma and allergic rhinitis in adult patients attending allergy clinics: ONEAIR study. J Invest Allerg Clin 2008;18:233-8. [ Links ]
31. Susanna Voltolini P M, Costantino Troise, Donatella Bignardi, Paola Modena, Daniele Arobba ACN. Trend of herbaceous pollen diffusion and allergic sensitisation in Genoa, Italy. Aerobiologia 2000;16:245-9. [ Links ]
32. Loureiro G, Rabaca MA, Blanco B, Andrade S, Chieira C, Pereira C. Urban versus rural environment any differences in aeroallergens sensitization in an allergic population of Cova da Beira, Portugal? Eur Ann Allergy Clin Immunol 2005;37:187-93. [ Links ]
33. Bonura A, Corinti S , Artale A, Di Felice G, Amoroso S, Melis M, et al. A hybrid expressing genetically engineered major allergens of the Parietaria pollen as a tool for specific allergy vaccination. Int Arch Allergy Imm 2007;142:274-84. [ Links ]
34. Tavares B, Machado D, Loureiro G, Cemlyn-Jones J, Pereira C. Sensitization to profilin in the Central region of Portugal. Sci Total Environ 2008;407:273-8. [ Links ]
35. Hrabina M PG, Van Ree R, Moingeon P. Grass pollen allergens. Clin Exp Allergy Rev 2008;8:7-11. [ Links ]
Ilda Abreu
Departamento de Biologia da Faculdade de Ciências da Universidade do Porto
Rua Campo Alegre
4169-007 Porto
E-mail: ianoronh@fc.up.pt
Data de recepção / Received in: 03/01/2013
Data de aceitação / Accepted for publication in: 11/03/2013














