Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Arquivos de Medicina
On-line version ISSN 2183-2447
Arq Med vol.23 no.2 Porto Mar. 2009
Is Starting First-line Anti-Retroviral Treatment Earlier Cheaper and Cost-effective?
Beck EJ, Mandalia S, Lo G, Baily G, Brettle R, Fisher M, Gompels M, Kinghorn G, McCarran B, Pozniak A, Tang A, Walsh J, Williams I, Youle M and Gazzard B for the NPMS-HHC Steering Group
Discussions on when to start anti-retroviral therapy (ART) have been debated for some time. Until recently the cut-off for starting ART was accepted to be above 200 cells/mm3 (1), while recent US and UK(2,3) guidelines now recommend starting ART with CD4 counts less than 350 cells/mm3. Since 2000, CD4+ cell counts at antiretroviral therapy initiation from industrialized countries has been stable at approximately 150-200 cells/mm3, which indicates that most people are starting therapy too late4. However, the CD4+ cell counts at treatment initiation have increased over time in sub-Saharan Africa and are now approaching levels similar to those observed in industrialized countries (4).
A recent UK study, covering the period 1996-2002, reported that regimens containing 2NRTIs + NNRTI were cost-effective compared with PI containing regimens for first-, second- and third-line HAART (5). Of people starting first-line ART at different CD4 strata, those presenting with lower CD4 counts were more likely to have presented late compared with those started ART at higher CD4 counts. (Kruskal-Wallis, p< 0.001; Table).
Table - Time between diagnosis and starting first-line ART, time-to-treatment failure and cost for treatment with first-line NNRTI and PI containing regimens in UK NPMS-HHC Sites 1996-2002 (2002 prices).
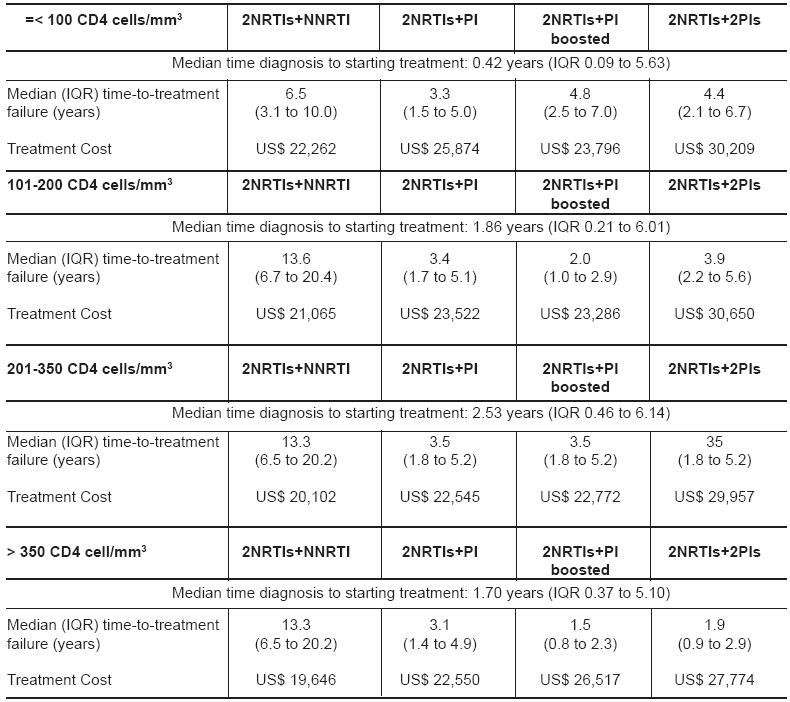
For all CD4 strata, the cost for first-line treatment till regimens failed, were less for those on 2NRTIs+NNRTI compared with PI containing regimens (Table). Cost for those who started on first-line 2NRTIs+NNRTI were greater if the CD4 count was less than or equal to 100 cells/mm3, compared when starting 2NRTIs+NNRTI with higher CD4 counts. This was also observed for the PI-containing regimens (Table).
Starting first-line therapy with 2NRTIs + NNRTI at a CD4 count between 101-200 cells/mm3 was cost-effective compared with starting 2NRTIS+NNRTI with less than 100 cells/mm3 and generated a cost of US$27,641 per life-year gained on first-line 2NRTIS+NNRTI. Starting 2NRTIS+NNRTI with a CD4 count of 201-350 produced a cost saving of US$10,562 per year compared when starting with a CD count between 101-200 cells/mm3, whereas starting with a CD4 count greater than 350 cells/mm3 compared with a CD4 count between 200-350 cells/mm3, produced a cost-saving of US$ 7,581 per year on 2NRTIS+NNRTI.
People starting ART with a CD4 count less than or equal to 100 cells/mm3 failed first-line therapy earlier compared with people starting ART with higher CD4 strata and people with the lower CD4 counts were most likely to be diagnosed late. 2NRTIs + NNRTI was less expensive and cost-effective compared with PI-based options and is a favoured regimen for first-line therapy. However, since 2002 new drugs have come on the market and these analyses need to be repeated taking these new regimens into consideration.
Earlier treatment with ART should not give rise to earlier resistance, aggravate resistance patterns or produce severe long-term adverse events. Furthermore, while cost at individual level might be less when starting with a higher CD4 count, this will however increase the number of people living with HIV and therefore increase the overall population cost of ART, which is particularly relevant for resource-limited countries. Analyses on the effectiveness, efficiency, equity and acceptability of drug regimens and other interventions need to be performed regularly and monitored over time. Countries need to develop their own HIV information systems to generate their own strategic information as healthcare systems and economies differ between countries.
REFERENCES
1 - Hogg RS, Yip B, Chan KJ et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA 2001;286:2568-77. [ Links ]
2 - BG Gazzard on behalf of the BHIVA. Treatment Guidelines Writing Group British HIV Association guidelines for the treatment of HIV-1-infected adults with antiretroviral therapy 2008. HIV Medicine 2008;9:563-608.
3 - Hammer SM, Eron JJ Jr, Reiss P, et al. Antiretroviral Treatment of Adult HIV Infection: 2008 Recommendations of the IAS-USA. JAMA 2008;300:555-70.
4 - Egger M. Outcomes of ART in Resource-limited and Industrialized Countries 14th Conference on Retroviruses and Opportunistic Illnesses, 27th February 2007, Abstract 62.
5 - Beck EJ, Mandalia S, Youle M, et al. Treatment Outcome and Cost-effectiveness of different HAART Regimens in the UK 1996-2002. Int J STD & AIDS 2008;19:297-304.














